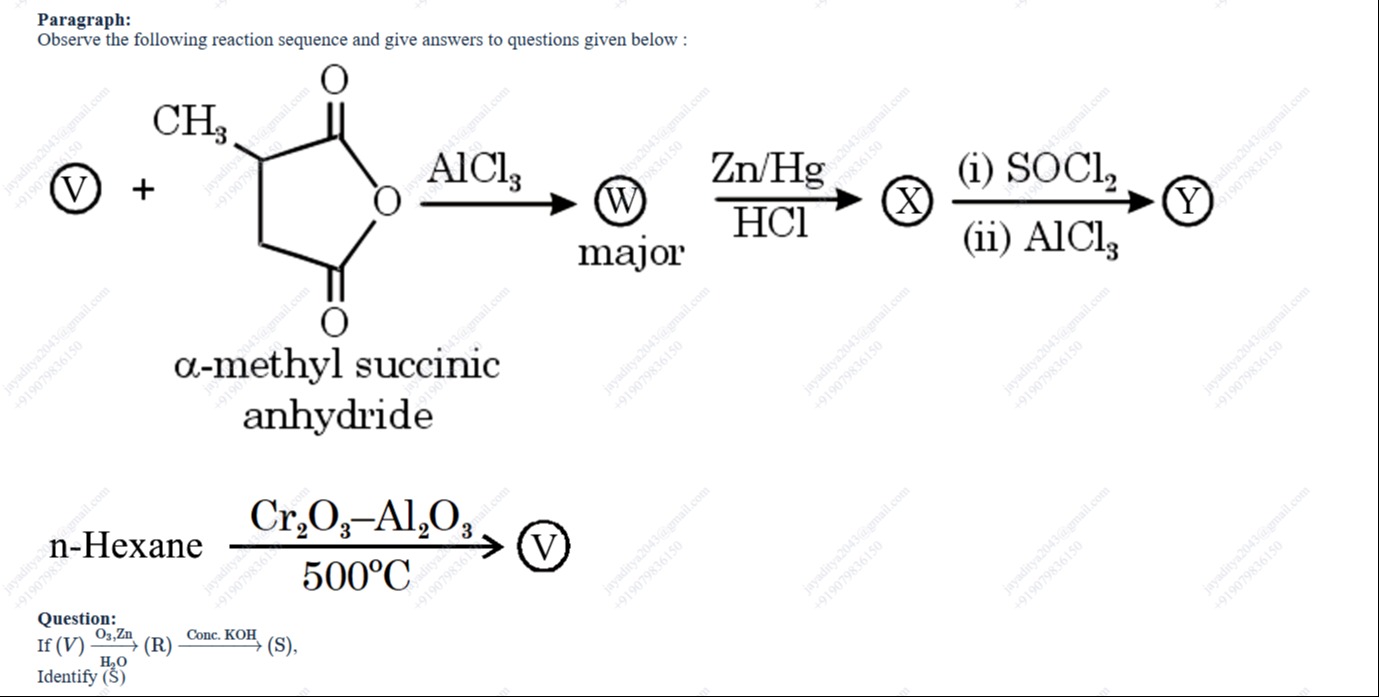
Question
Question: If (V) $\xrightarrow[H_2O]{O_3,Zn}$ (R) $\xrightarrow[]{Conc. KOH}$ (S), Identify (S)...
If (V) O3,ZnH2O (R) Conc.KOH (S), Identify (S)
Muconic acid (2E,4E–hexadienedioic acid).
Solution
Solution Explanation:
-
Identification of V:
From the overall scheme, V is obtained from n‐hexane over Cr₂O₃–Al₂O₃ at 500°C. In such catalytic reforming reactions, n‐hexane is converted into an aromatic compound. Hence, V is benzene. -
Ozonolysis of Benzene:
BenzeneO3,Zn/H2OOHC–(CH=CH)2–CHO(R)
When benzene is treated with O₃ and then reduced with Zn/H₂O, the aromatic ring is cleaved completely. This gives the intermediate dialdehyde known as muconaldehyde: -
Treatment with Conc. KOH:
The intermediate dialdehyde (R), having non‐enolizable aldehyde groups, undergoes a base–induced Cannizzaro reaction. Both aldehyde groups are converted into carboxylate groups. Acid work–up (if needed) furnishes (2E,4E)‐hexadienedioic acid, commonly known as muconic acid (S).
Thus, (S) is muconic acid.
