Question
Question: Paragraph: Observe the following reaction sequence and give answers to questions given below : n-He...
Paragraph: Observe the following reaction sequence and give answers to questions given below :
n-Hexane Cr2O3−Al2O3500∘C (V)
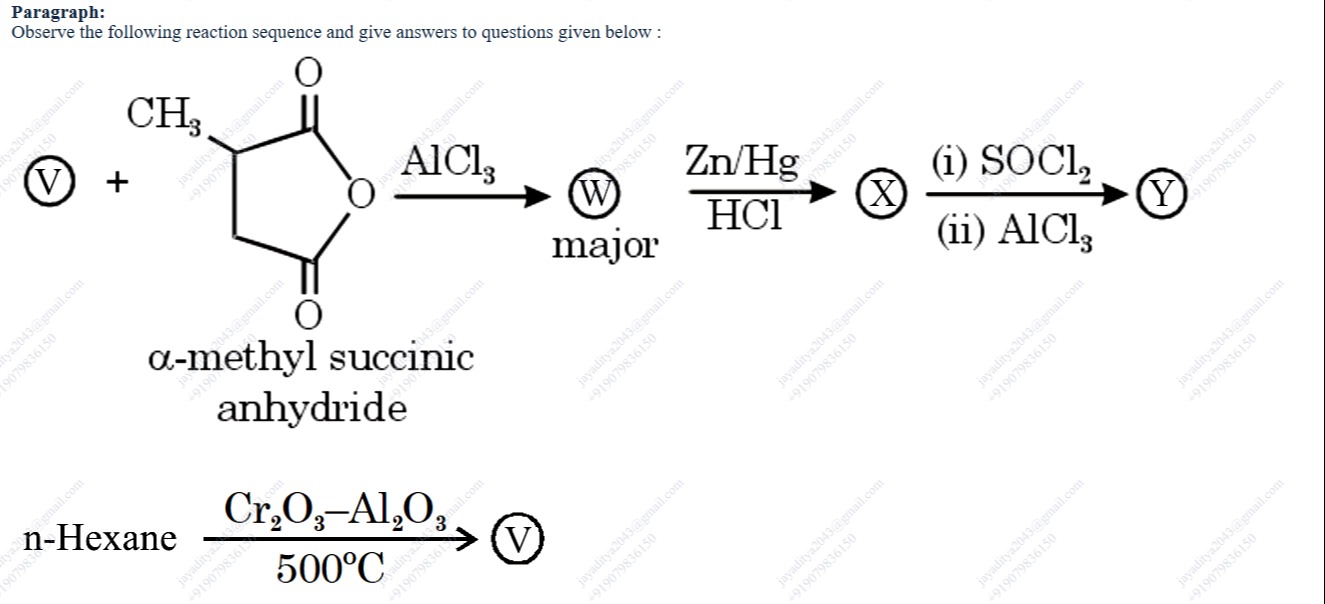
- (V) = Benzene
- (W) = Ph–CO–CH(CH₃)–CH₂COOH [2–(1–oxopropyl)benzoic acid]
- (X) = Ph–CH₂–CH(CH₃)–CH₂COOH [its Clemmensen reduced derivative]
- (Y) = 1–methyl–2,3–dihydro–1H–inden–1–one
Solution
The reaction sequence converts n–hexane into benzene, then via a Friedel–Crafts acylation with a dicarbonyl reagent, into an acyl–benzoic acid which upon reduction (Clemmensen) and then intramolecular cyclization gives a substituted indanone.
Step 1. Aromatization:
n–Hexane is passed over a Cr₂O₃–Al₂O₃ catalyst at 500 °C. Under these conditions the alkane is dehydrogenated and cyclized to give benzene. Thus, (V) = benzene.
Step 2. Friedel–Crafts Acylation with α–methylsuccinic anhydride:
The reagent “α–methylsuccinic anhydride” is a five–membered anhydride having two carbonyl groups with a –CH₃ on the “alpha” carbon. In the presence of AlCl₃ one of the acyl groups is “liberated” as its acylium ion. Therefore, the benzene ring is acylated giving an intermediate whose structure may be written as Ph–CO–CH(CH₃)–CH₂COOH. We denote this product as (W) = 2–(1–oxopropyl)benzoic acid.
Step 3. Clemmensen Reduction:
Treatment of (W) with Zn/Hg in HCl (Clemmensen reduction) reduces the carbonyl group of the ketone (–CO–) to a methylene (–CH₂–) group while leaving the –COOH intact. That is, Ph–CO–CH(CH₃)–CH₂COOH → Ph–CH₂–CH(CH₃)–CH₂COOH. This product is labeled (X).
Step 4. Cyclization via Acid Chloride Formation and Intramolecular Friedel–Crafts Reaction:
On treatment with SOCl₂ the carboxylic acid is converted into the corresponding acid chloride. Then in the presence of AlCl₃ an intramolecular Friedel–Crafts acylation takes place. The acid chloride group is “attacked” by the aromatic ring and the two parts become fused giving a five–membered ring on the benzene. In short, the sequence yields (Y) = 1–methyl–2,3–dihydro–1H–inden–1–one.
