Question
Question: Paragraph for Questions 1 and 2 A 0.60 kg sample of water and a sample of ice are placed in two comp...
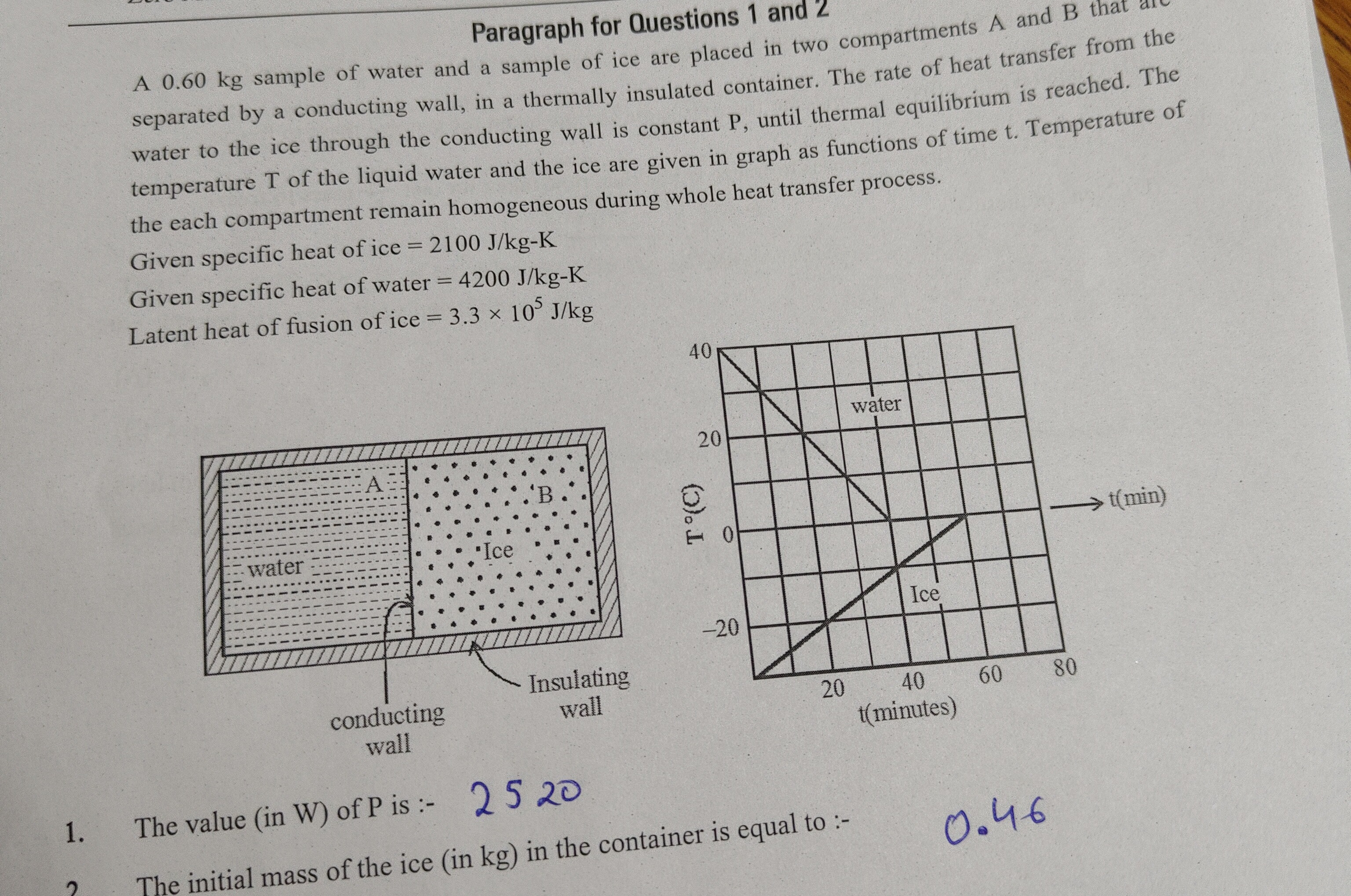
Paragraph for Questions 1 and 2 A 0.60 kg sample of water and a sample of ice are placed in two compartments A and B that are separated by a conducting wall, in a thermally insulated container. The rate of heat transfer from the water to the ice through the conducting wall is constant P, until thermal equilibrium is reached. The temperature T of the liquid water and the ice are given in graph as functions of time t. Temperature of the each compartment remain homogeneous during whole heat transfer process.
Given specific heat of ice = 2100 J/kg-K Given specific heat of water = 4200 J/kg-K Latent heat of fusion of ice = 3.3 × 10⁵ J/kg
- The value (in W) of P is :-
- The initial mass of the ice (in kg) in the container is equal to :-
25
20
42
0.46
42
Solution
The rate of heat transfer P can be calculated from the heat lost by the water as it cools from 40∘C to 0∘C in 40 minutes. Mass of water, mw=0.60 kg. Specific heat of water, cw=4200 J/kg-K. Temperature change of water, ΔTw=40∘C−0∘C=40 K. Time taken, Δt=40 minutes =40×60 seconds =2400 seconds.
The heat lost by the water is: ΔQw=mw×cw×ΔTw ΔQw=0.60 kg×4200 J/kg-K×40 K=100800 J
The rate of heat transfer P is: P=ΔtΔQw=2400 s100800 J=42 W
For the second part of the question, we use the calculated rate of heat transfer P=42 W. The ice warms from −20∘C to 0∘C in 40 minutes. Specific heat of ice, ci=2100 J/kg-K. Temperature change of ice, ΔTi=0∘C−(−20∘C)=20 K. Time taken, Δt=2400 seconds.
The heat gained by the ice is: ΔQi=mi×ci×ΔTi Where mi is the initial mass of ice.
The rate of heat transfer P is also equal to the heat gained by the ice divided by the time: P=ΔtΔQi 42 W=2400 smi×2100 J/kg-K×20 K 42=2400mi×42000 mi=4200042×2400=422.4×42=2.4 kg
Note: There is a discrepancy with the handwritten answers provided in the original problem. The solution above is derived from a consistent application of physics principles to the given graph and parameters. The question asks for the value of P, which is 42 W.
