Question
Question: Packing fraction in 2D-hexagonal arrangement of identical sphere is: A. \(\dfrac{\pi }{{3\sqrt 2 }...
Packing fraction in 2D-hexagonal arrangement of identical sphere is:
A. 32π
B. 33π
C. 23π
D. 6π
Solution
packing fraction is defined as the fraction of the volume in the crystal structure that is occupied by the constituent particle. It is also called packing efficiency or atomic packing factor. This definition is of crystallography.
Complete answer:
For 2D-hexagonal close packing system:
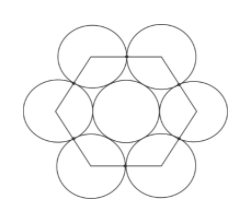
2r=a(from diagram)
As we have seen in the figure there is one hexagon which can divide in the 6 equilateral triangles.
So to find the area of the hexagon we will divide the area in 6 equilateral triangles.
So as we all know that area of an equilateral triangle is 43a2, where a is the side of the hexagon or side of the triangle.
So area of hexagon = 6 × area of equilateral triangle = 6×43a2
Now we have a=2r
So putting the value of a we get
Total area of hexagon will be 6×43(2r)2=6×43×4r2=63r2
Now from the figure we can say that total spherical constituent in the hexagon will be-
1+31×6=3(one sphere of the middle and one third of 6 sphere)
Let radius of one sphere is r
So area of the 3 sphere is 3πr2
∴Packing efficiency of Packing fraction = Total area of the hexagonTotal occupied space
Packing efficiency of Packing fraction = 63r23πr2=23π
**Hence option C will be the correct answer.
Note:**
Packing efficiency is the term used In the crystallography. This term is used to define the solidness of an atom. If packing efficiency is more than the atom will be solid and if packing efficiency is less than the atom less solid.
