Question
Question: p-Nitrophenol is a stronger acid than phenol while p-cresol is a weaker acid. This can be explained ...
p-Nitrophenol is a stronger acid than phenol while p-cresol is a weaker acid. This can be explained as:
A. - CH3 group decreases the electron density on the oxygen of O-H group making p-cresol a weaker acid.
B. - NO2 decreases the electron density on the oxygen of O-H group making p-nitrophenol a stronger acid.
C. - CH3 group increases the electron density on the oxygen of O-H group making the release of H + easier
D. - NO2 group increases the electron density on oxygen of O-H group making release of H + easier
Solution
The p-nitrophenol is a nitro group attached to the para position of phenol. Similarly, p-cresol is a methyl group attached to the para position of phenol. An electronegative atom pulls the electrons and has high electron density. The presence of the electron-withdrawing group and electron-donating group affects the acidity of the compounds. Acidity is determined by the ability to release H + ions.
Complete step by step answer:
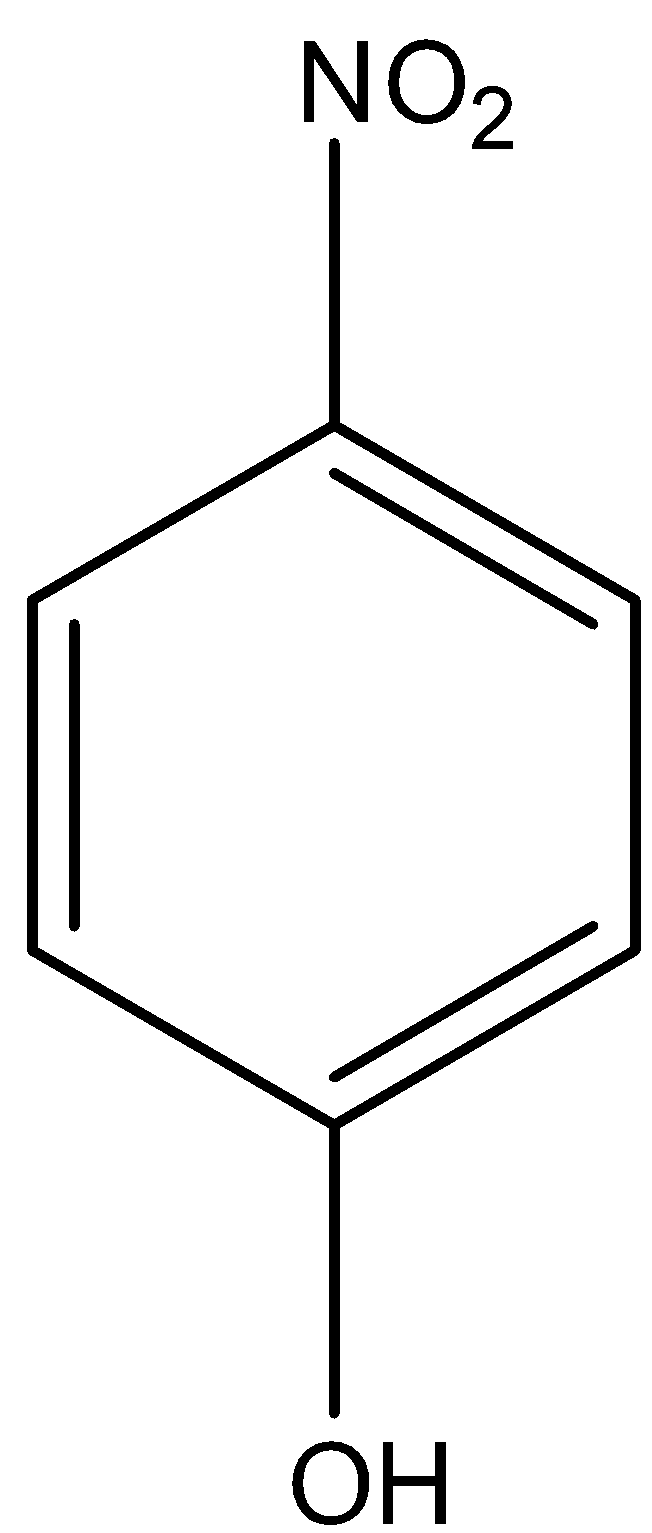
Let us look at the structure of p-nitrophenol. Phenol is an electron-deficient group.
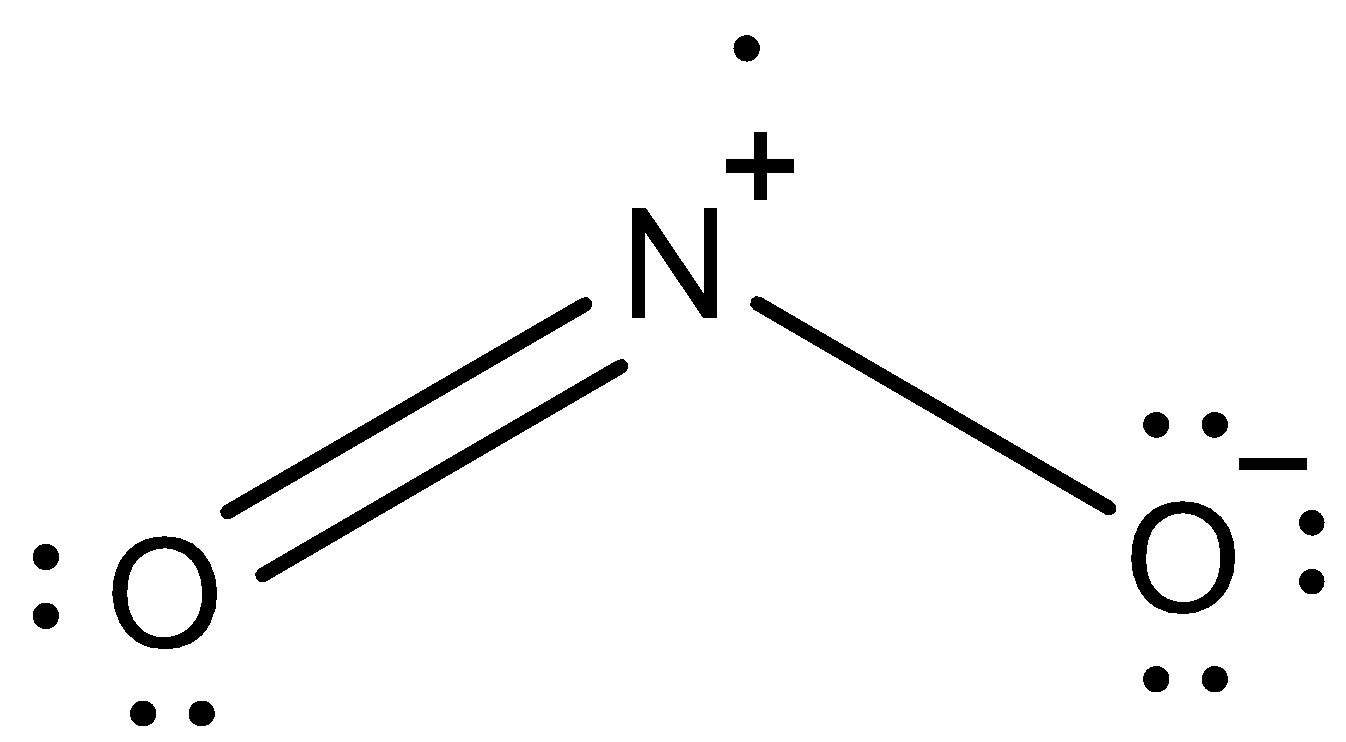
This is the lewis dot structure of NO2.
We can see that Nitrogen in NO2 already has a positive charge on it. It is because it gave one of its lone pairs to oxygen (double bond). When this nitrogen is attached to the benzene ring, the nitrogen will pull the electrons from the benzene ring to reduce the positive charge.
Therefore, NO2 is an electron-withdrawing group.
In p-nitrophenol, since it is NO2 is an electron-withdrawing group, all the electron density will be drifted towards NO2
This will result in a decrease in the electron density of oxygen in the O-H bond.
Thus, the release of H + is easier.
Now, let us look at o-cresol.
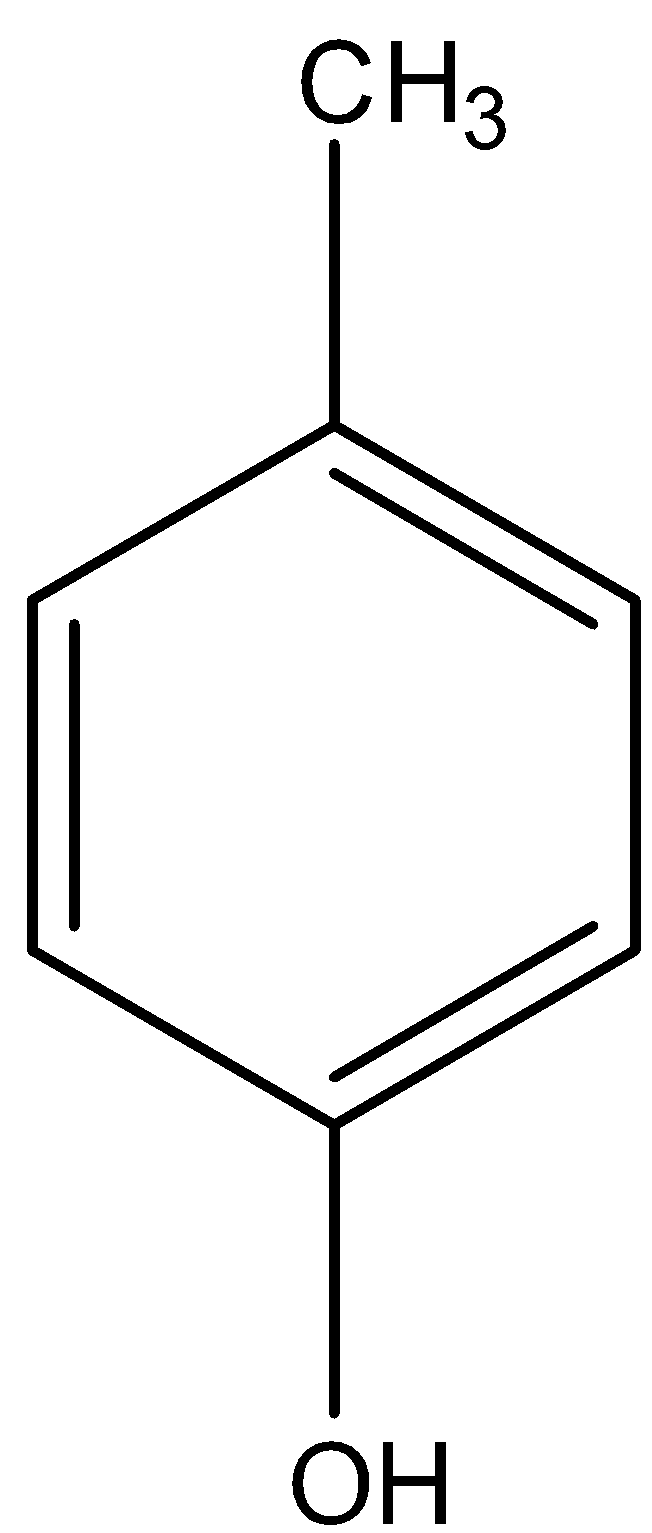
The - CH3 is attached to the para position of phenol.
We know that the methyl group in cresol is electron-donating. It gives its electron density to the electron-deficient phenol. Thus, the electron density on the O-H group increases.
Therefore, it cannot give out H + ions.
Therefore, the correct answer is that the - NO2 decreases the electron density on the oxygen of the O-H group making p-nitrophenol a stronger acid.
Thus, the correct option is (B).
Note: The presence of an electron-donating group will decrease the acidity. This is because the electron-donating group increases the electron density on the electron-deficient species. Thus, it makes it difficult to release H + ions as the bonds are stronger. The presence of electron-withdrawing groups - NO2 decreases the electron density and facilitates the release of H + ions. Other examples of electron-donating groups are - NH2, -NR, - etc., and the electron-withdrawing groups are halogen, - NO2, - CF3
