Question
Question: P in \[PC{l_5}\] has \[s{p^3}\] hybridization. Which one of the following statement is wrong about \...
P in PCl5 has sp3 hybridization. Which one of the following statement is wrong about PCl5 structure?
A.Two P−Cl bonds are stronger and three P−Cl bonds are weaker.
B.Two P−Cl bonds are axial and larger than three P−Cl equatorial bond.
C.PCl5has trigonal bipyramidal geometry with non- polar nature.
D.All the above statements are wrong.
Solution
The VSEPR theory can be used to predict the shape from the hybridization. The number of types of bonds present in phosphorus pentachloride is two which is axial bond and equatorial bond. And the hybridization of PCl5 is sp3. Which means, two electrons are present in 3s orbital and three electrons are present in 3p orbital. The shorter bond is always stronger than the longer bond.
Complete answer:
Among the following statements, the first option, “Two P−Cl bonds are stronger and three P−Cl bonds are weaker “is a wrong statement. Because, the number of equatorial bonds present in PCl5 is three and the number of axial bonds is two. And the axial bonds are longer than equatorial bonds. We can draw the structure of PCl5,
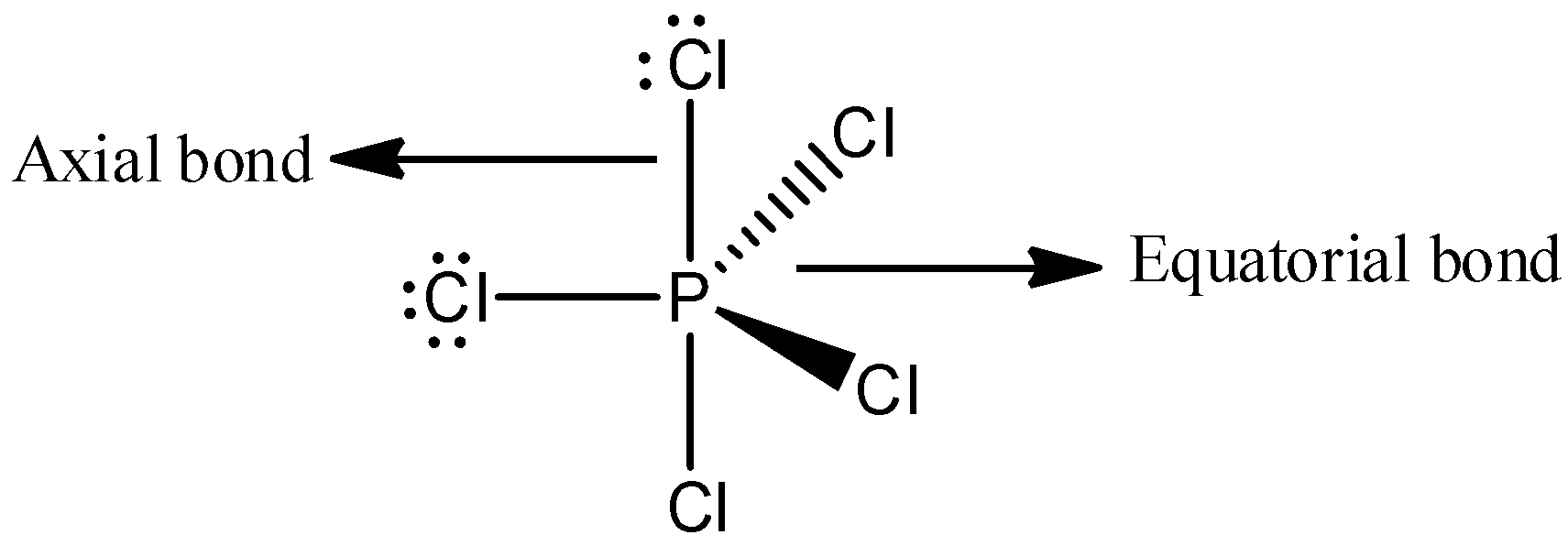
The bond length is always inversely proportional to the bond strength. Therefore, when the bond length is increased, its bond strength will decrease and here, the two P−Cl are weaker than three P−Cl bonds. Thus, we can say that the statement, “Two P−Cl bonds are stronger and three P−Cl bonds are weaker” is wrong.
Hence, option (A) is correct.
Two P−Cl bonds are axial and larger than three P−Cl equatorial bonds is a correct statement. Hence, the option (B) is incorrect.
The statement, PCl5 has trigonal bipyramidal geometry with non- polar nature is correct. Let’s draw the structure,
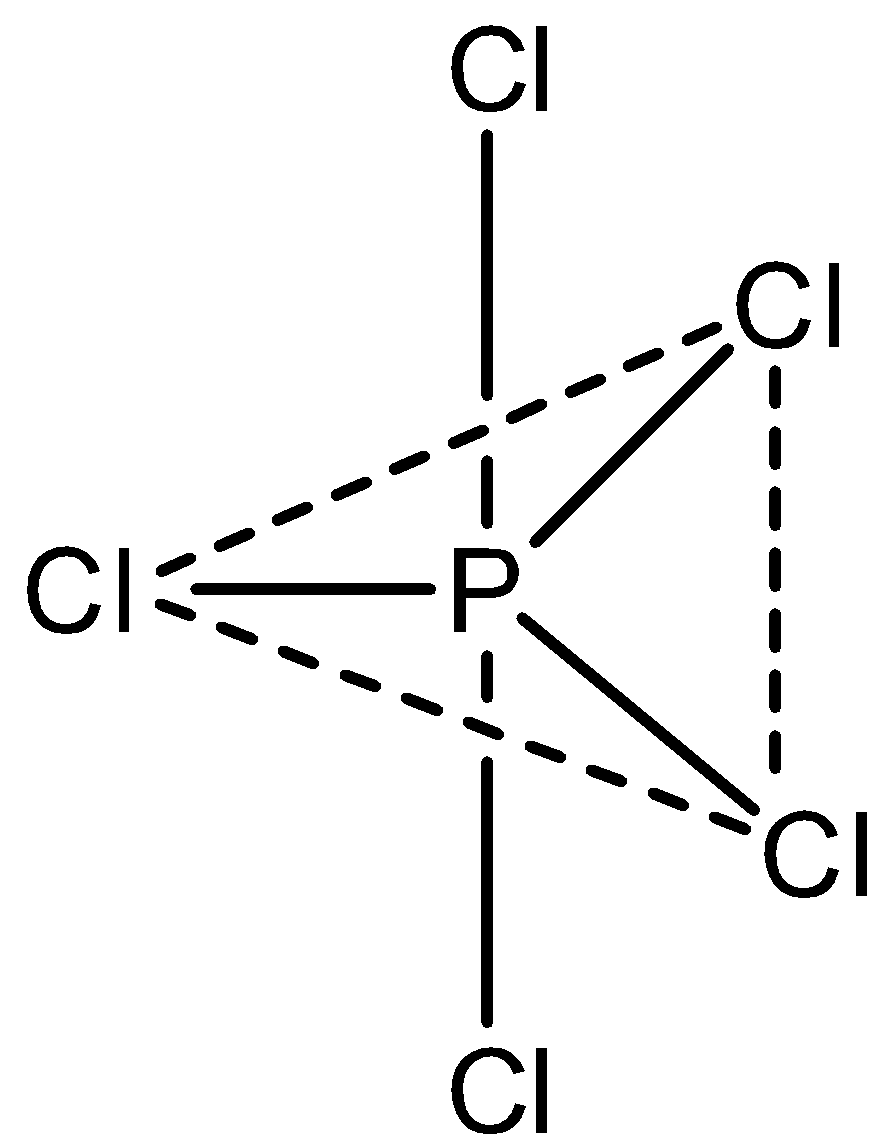
Hence, option (C) is incorrect.
Only the first statement is wrong and the other two statements are correct. Hence, the option (D) is incorrect.
Note:
Among the given statements, two P−Cl bonds are stronger and three P−Cl bonds are weaker is a wrong statement. Because, the axial bond present in phosphorus pentachloride is always weaker than the equatorial bond. There are two equatorial bonds present in PCl5 and that two bonds are weaker than the other three bonds. And the remaining two statements are true about PCl5 structure.
