Question
Question: p-cresol reacts with chloroform in an alkaline medium to give the compound \({\text{A}}\)which adds ...
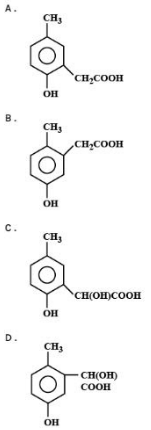
p-cresol reacts with chloroform in an alkaline medium to give the compound Awhich adds hydrogen cyanide to form the compound B. The latter on acidic hydrolysis gives chiral carboxylic and ′C’which is:
Solution
Hint- After reading this above problem carefully we can think of a famous chemical reaction called Reimer-Tiemann. Reimer-Tiemann reaction is the reaction used to convert the phenol into an ortho hydroxy benzaldehyde using the chloroform and a strong base.
Complete step by step solution:
Given problem is depicting the condition as follows,
p - CH3C6H5OH + CHCL3 + NaOH→AHCNBH + /H2ORCOOH(chiral acid)
The Reamer-Tiemann reaction gives the CHO group at ortho and OH group on the benzene ring.
Hence the compound A will be as follows,
A = pCH3(o - CHO)C6H3OH
now after the formation of the compound a as above the addition of HCNat the aldehyde group will result in cyanohydrin formation, written as follows,
B = pCH3(o - CH(CN)(OH))C6H3OH
After this the hydrolysis is taking place, on hydrolysis, the cyanide group will give COOHgroup as
B = pCH3(o - CH(COOH)(OH))C6H3OH
Hence finally we have got our solution,
So, after the sequential reaction the resultant compound of the above chemical reaction which is being governed by the Riemer- Tiemann reaction.
Hence option (A) is the correct answer.
Note- To check the chirality of any compound that means to check that the given compound is chiral or not, we have to look for the carbons with four different groups attached to identify potential chiral centres. We have to draw the structural formula for the molecule of the compound and the mirror image of that particular structure of the molecule or compound.If the compound or thr molecule in the mirror image is the same as that of the structural representation of that compound, then the compound is not a chiral compound.
From the above discussion we have come to the result that if the structural formula of a molecule of a cannot superpose the mirror image by any combination of translation and rotation then it will be the chiral molecule of that compound.
