Question
Question: \( {P_4}{O_6} \) reacts with water to give: (A) \( {H_3}P{O_3} \) (B) \( {H_2}{P_2}{O_7} \) ...
P4O6 reacts with water to give:
(A) H3PO3
(B) H2P2O7
(C) HPO3
(D) H3PO4
Solution
Phosphorus trioxide reacts with hydrogen chloride to give phosphorous trichloride and phosphoric acid. In the given question we need to know the product formed when the P4O6 reacts with water.
Complete step by step answer:
P4O6 reacts with water to give phosphoric acid.
The chemical name of P4O6 is Phosphorus trioxide.
The structure is:
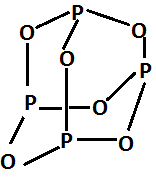
It is formed by burning phosphorus in the limited supply of oxygen. The reaction is as follows:
P4+3O2→P4O6
This product reacts with water and forms phosphorus acid. The chemical formula of phosphoric acid is H3PO3 The structure is:
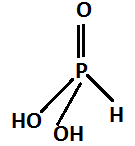
The reaction occurs as follows: P4O6+6H2O→4H3PO3
Hence, option A. H3PO3 is the correct answer to the given question.
Additional information:
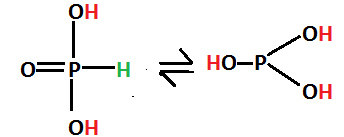
H3PO3 exists in tautomeric form because they are readily convertible and the former is called phosphonic acid whereas the latter is called phosphoric acid.
Note:
Uses of H3PO3 :
For the production of basic lead phosphite. It is used as a stabilizer in Polyvinyl chloride and other polymers containing chlorine.
As a reducing agent: it is a strong reducing agent which is also used in production of synthetic fibers and pesticides.
As a ligand: with the metals of d6 configuration, it acts as a ligand by forming a co-ordinate bond with those metals.
