Question
Question: Oxymercuration-demercuration reaction \[{\text{Hg(OAC}}{{\text{)}}_{\text{2}}}{\text{/THF - }}{{\tex...
Oxymercuration-demercuration reaction Hg(OAC)2/THF - H2O followed by NaBH4,C2H5OH)on CH3CH = CH2produces:
A)CH3CH2CH2CO
B) CH3CH(OH)CH3
C) CH3CH(OH)CH2OH
D) CH3COCH3
Solution
Oxymercuration-demarcation reaction is an alternative method of hydration of alkene. Given alkene is an asymmetric alkene. Additions take place according to Markovnikov’s rule. The first step of the reaction is the formation of cyclic mercurinium ions. The second step is the opening of mercurinium ion by the nucleophilic attack of water. Finally, demercuration takes place by reduction with NaBH4 the reagent.
Complete solution:
As we know that Oxymercuration-demercuration reaction is a hydration reaction so the product of the reaction will be alcohol.Hence, Option (A) and (D) are the incorrect answers.
Now we will see the detail mechanism of the Oxymercuration-demercuration reaction.
Step1: Formation of cyclic mercurinium ion: Nucleophilic attack of π electrons of C = Con electrophilic Hg and loss of an acetate ion.
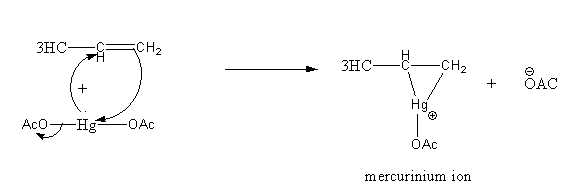
Step2: Opening of mercurinium ion by nucleophilic attack of water. Attack of water takes place according to Markovnikov’s rule, that is it attacks on the more substituted carbon atom.
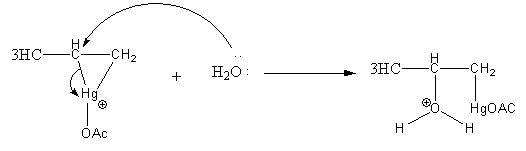
Step3: Deprotonation of oxonium ion by base acetate ion.
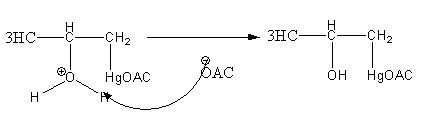
Step4: Breaking of C - Hg bond and formation of C - H by reduction withNaBH4.
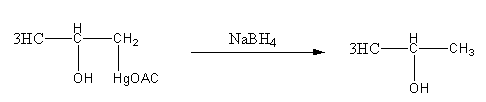
Thus, option (D) is incorrect as the product is not diol.
Thus, the correct options are (B).
Note: Oxymercuration-demercuration reaction involves the transformation of C = C to OH - C - C - H bond. More substituted alcohol is the major product. It is an electrophilic addition type of reaction.
