Question
Question: Oxygen molecule is formed by: A.One axial s-s overlap and one p-p axial overlap B.Two p-p axial ...
Oxygen molecule is formed by:
A.One axial s-s overlap and one p-p axial overlap
B.Two p-p axial overlap
C.Two p-p sidewise overlap
D.One p-p axial and one p-p sidewise overlap
Solution
Hint: Each oxygen atom has three p orbitals which are perpendicular to each other and hence can overlap axially as well as sidewise.
Complete step by step answer:
The electronic configuration of Oxygen is 1s2 2s2 2px2 2py1 2pz1 .
So, p orbitals of a single oxygen atom would look like –
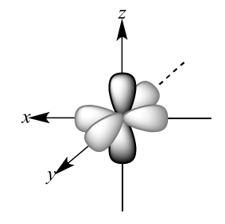
All the 2p orbitals are perpendicular to each other. So, 2pz orbitals of each oxygen atom will overlap sidewise to form a sigma bond. 2py orbitals of each oxygen would overlap axially forming a pi bond. So, a double bond will be formed between the two oxygen atoms, out of which one will be pz - pz sigma bond and the other will be py - py pi bond. So, the structure of the molecule will be –
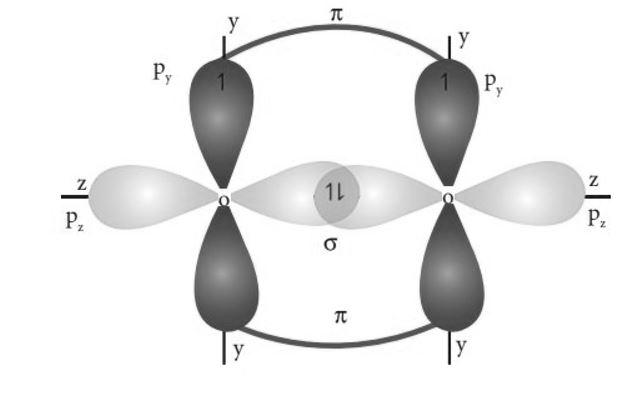
Hence, option D is correct.
Additional information: Oxygen is the most abundant available element in the earth. It is denoted by the symbol O and is categorized under the chalcogen group in the periodic table. It is colourless, odourless, tasteless gas which easily dissolves in water. It is essential for survival of human beings.
It is used in the production and manufacturing of glass and stone products, and in mining. Special oxygen chambers are used in case of high pressure to increase the partial pressure of oxygen around the patient. The primary applications of oxygen include melting, refining, and manufacture of steel along with other metals.
Note: Oxygen requires two electrons to complete its octet. It shares these two electrons with another oxygen atom and hence always exists as a diatomic molecule.
