Question
Question: Oxidation numbers of chlorine atoms in \(CaOC{l_2}\) are A. 0,0 B. -1, -1 C. -1, +1 D. none ...
Oxidation numbers of chlorine atoms in CaOCl2 are
A. 0,0
B. -1, -1
C. -1, +1
D. none of these
Solution
In bleaching powder CaOCl2, two chlorine atoms are present. One chlorine atom is attached to the calcium atom directly and other chlorine is attached to the oxygen atom.
Complete step by step answer:
The oxidation number is also known as the oxidation state. It is defined as the overall electron in which an atom either accepts or donates to the neighboring atom to generate a chemical bond with the surrounding atom.
The compound CaOCl2 is commonly known as bleaching powder, the chemical name is calcium hypochlorite and is written as Ca(OCl)Cl in which two chlorine atoms with different oxidation states are attached.
The structure of CaOCl2 is shown below
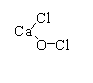
The Ca(OCl)Cl can be written as [Ca+2(OCl)−1Cl−1]
The oxidation state of calcium is +2.
The oxidation state of chlorine attached to calcium is -1.
As we know the oxidation state of oxygen is -2 and the oxidation state of the overall molecule in (OCl) is -1, so the resulting oxidation state of chlorine will be +1. The sum of all the charges should be equal to zero as the compound is neutral.
Thus, the oxidation number of chlorine atoms in CaOCl2 is -1 and +1.
Thus, the correct answer is option C.
Note:
The sum of the oxidation state in the polyatomic ion is the same as the charge on the ion but in the compound, CaOCl2 no charge is present as it is a neutral compound.
