Question
Question: Oxidation number of sulphur in peroxomonosulphuric acid \(({H}_{2}S{O}_{5})\) is : A. +4 B. +2 ...
Oxidation number of sulphur in peroxomonosulphuric acid (H2SO5) is :
A. +4
B. +2
C. +6
D. -2
Solution
The oxidation state or oxidation number is defined as the no. of electrons lost or gained by the atom of that element in the compound. It is used to keep track of how many electrons an atom has.
Complete step by step answer:
The oxidation states of sulphur are -2, -1, 0, +1, +2, +3, +4, +5, and +6. It is strongly acidic oxide. But generally, Sulphur shows only -2, 0, +2, +4 and +6 oxidation states. peroxo acids of sulphur are those acids which have a O - O bond in them. Let us talk about peroxomonosulphuric acid. It is also known as the persulphuric acid, peroxysulphuric acid, or Caro's acid. In this acid, the central sulphur adopts its tetrahedral geometry. The molecular formula of peroxomonosulphuric acid can be written as HO−O−S(O2)−OH. It is one of the strongest oxidants known. The oxidation state of sulphur is peroxomonosulphuric acid is +6. The oxidation state of sulphur in H2SO5 is evident from the following structure. The two oxygen atoms attached to the sulphur with double bonds are known as peroxides and have the oxidation state -1. And the other two oxygen atoms attached to sulphur with single bonds have the oxidation state -2. Now to form a covalent bond with these atoms sulphur has to donate its six electrons to the oxygen atoms. And therefore, the oxidation state of sulphur is +6.
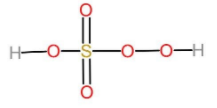
Therefore, the correct option is option (C).
Peroxomonosulphuric acid is used for treatment of swimming pools and denature cleaning. Alkali salts of peroxomonosulphuric acids are used for delignification of wood. It is also used in laboratories.
Note: Students tend to confuse between H2SO5 and H2S2O5. Please see that these two compounds are totally different from each other. The oxidation state of sulphur in H2SO5 is +6 whereas, H2S2O5 has two sulphur atoms and thus has two different oxidation states i.e., +3 and +5.
