Question
Question: Oxidation number of Cr in \(Cr{O_5}\) is: \( A) + 10 \\\ B) + 6 \\\ C) + 4 \\\ ...
Oxidation number of Cr in CrO5 is:
A)+10 B)+6 C)+4 D)+5
Solution
We can define oxidation state as oxidation degree (loss of an electron) in a chemical compound. We can calculate the oxidation state by an element in a compound by using the rules of oxidation numbers.
Complete step by step answer:
We know that oxidation state is the loss of an electron in a chemical compound. We can now see a few rules of oxidation numbers.
-A free element will be zero as its oxidation number.
-Monatomic ions will have an oxidation number equal to charge of the ion.
-In hydrogen, the oxidation number is + 1, when combined with elements having less electronegativity, the oxidation number of hydrogen is -1.
-In compounds of oxygen, the oxidation number of oxygen will be -2 and in peroxides, it will be -1.
-Group 1 elements will have +1 oxidation number.
-Group 2 elements will have +2 oxidation numbers.
-Group 17 elements will have -1 oxidation number.
-Sum of oxidation numbers of all atoms in neutral compounds is zero.
-In polyatomic ions, the sum of the oxidation number is equal to the ionic charge.
We can draw the structure of CrO5 as,
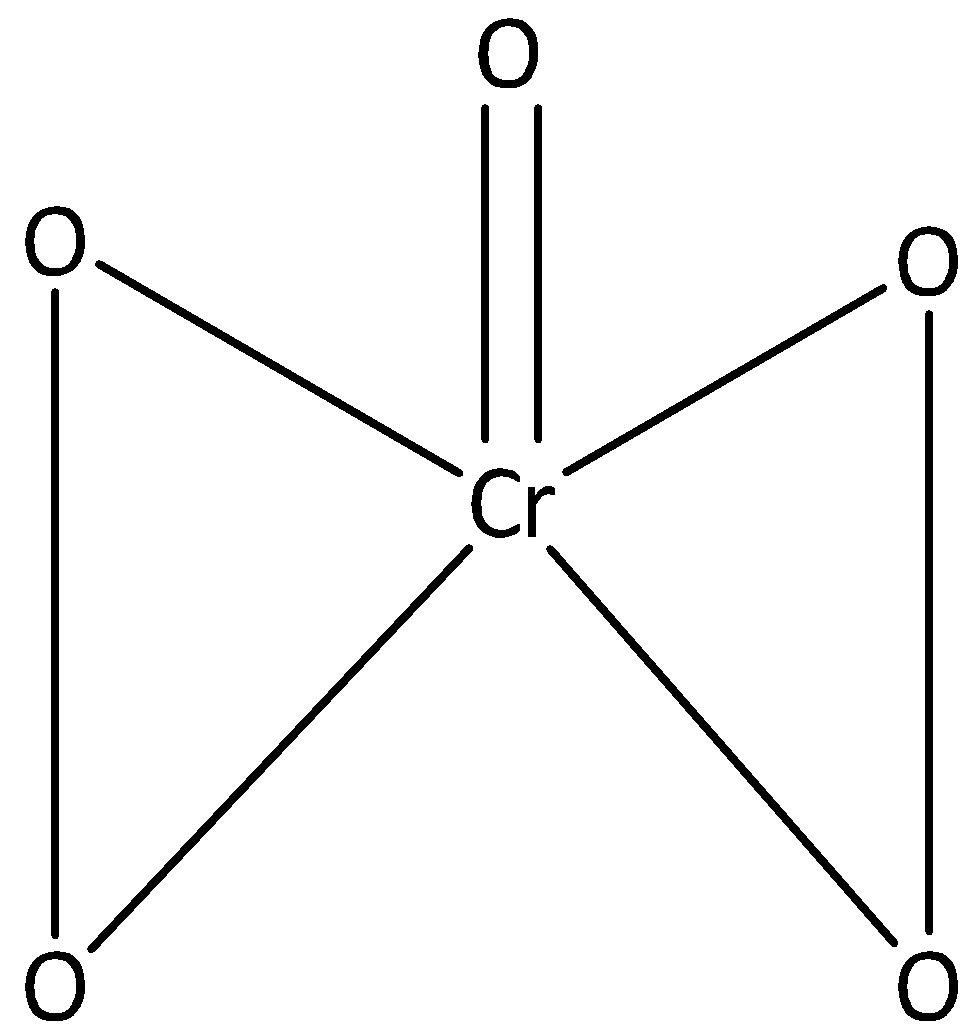
In CrO5 one atom of oxygen is linked to chromium through the double bond, and the oxidation number is -2. The remaining four oxygen atoms are linked to chromium through a single bond, and their oxidation number is -1.
Let us now calculate the oxidation state of Cr in CrO5.
Let x be the oxidation number of Cr.
x+1(−2)+4(−1)=0 x=+6
The oxidation number of Chromium in CrO5 is + 6.
Hence, Option B is the correct answer.
Note:
We must know that the four of the oxygen atoms present in CrO5 exists as peroxides, and therefore their oxidation number is - 1.
