Question
Question: Figure shows a cylindrical tube of radius 5cm and length 20cm. It is closed by tight fitting cork. T...
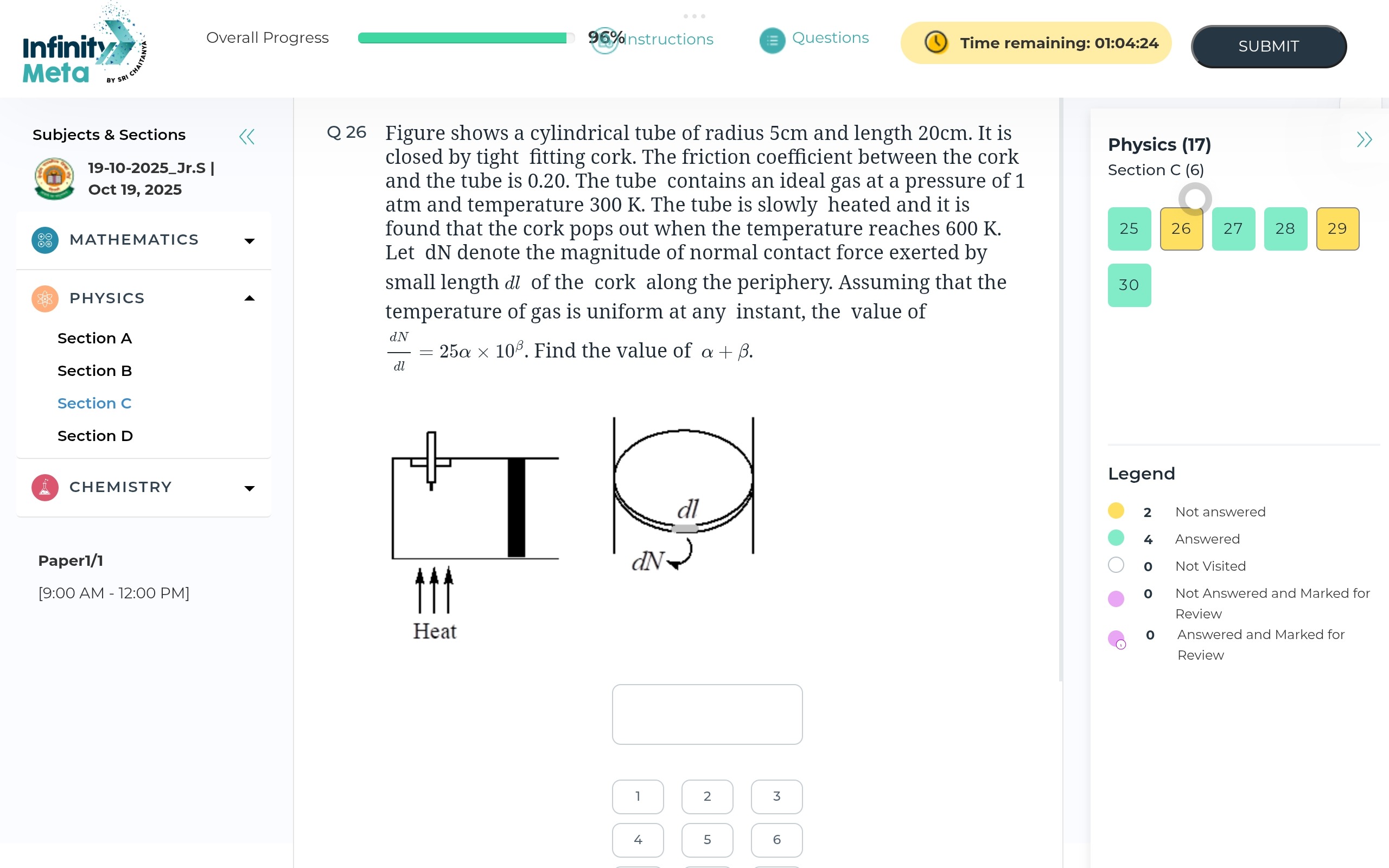
Figure shows a cylindrical tube of radius 5cm and length 20cm. It is closed by tight fitting cork. The friction coefficient between the cork and the tube is 0.20. The tube contains an ideal gas at a pressure of 1 atm and temperature 300 K. The tube is slowly heated and it is found that the cork pops out when the temperature reaches 600 K. Let dN denote the magnitude of normal contact force exerted by small length dl of the cork along the periphery. Assuming that the temperature of gas is uniform at any instant, the value of dldN=25α×10β. Find the value of α+β.
4.6
4.16
3.6
1.6
4.6
Solution
The final pressure P2 when the temperature reaches T2=600 K can be found using Gay-Lussac's Law, as the volume is constant: P2=P1T1T2 Given P1=1 atm and T1=300 K, T2=600 K: P2=1 atm×300 K600 K=2 atm The normal contact force dN exerted by a small length dl of the cork along the periphery is due to the internal pressure P2 acting on the area element dA. This area element can be approximated as dA=L×dl, where L is the length of the cork and dl is the arc length along the periphery. Thus, the force dN is given by: dN=P2×dA=P2×L×dl The quantity dldN is therefore: dldN=P2×L We are given the radius r=5 cm and length L=20 cm=0.20 m. We need to convert the pressure to Pascals: 1 atm≈1.013×105 Pa. So, P2=2 atm≈2×1.013×105 Pa=2.026×105 Pa. Now, calculate dldN: dldN=(2.026×105 Pa)×(0.20 m)=0.4052×105 N/m=4.052×104 N/m We are given that dldN=25α×10β. So, 4.052×104=25α×10β. To find integer values for α and β that fit the format, we can approximate P2 to 2×105 Pa. Then, dldN=(2×105 Pa)×(0.20 m)=0.4×105 N/m=4×104 N/m. Now, 4×104=25α×10β. Let's choose β=3. 4×104=25α×103 40=25α α=2540=58=1.6. Therefore, α=1.6 and β=3. The value of α+β=1.6+3=4.6.
