Question
Question: Outline the reaction sequence for the conversion of ethene to ethyne (the number of steps should not...
Outline the reaction sequence for the conversion of ethene to ethyne (the number of steps should not be more than two).
A. A:HOCl B: KOH
B. A: bromine water B: Diethylamine
C. A:Cl2,hv B:Ca(OH)2
D. A: bromine water B: KOH
Solution
We have to perform mechanisms or perform the steps by the reagents given to achieve our product. Ethene has two carbon atoms with a double bond and rest vacancies are filled by hydrogen atoms. Ethyne has two carbon atoms with a triple bond and rest vacancies are filled by hydrogen atoms.
Complete answer:
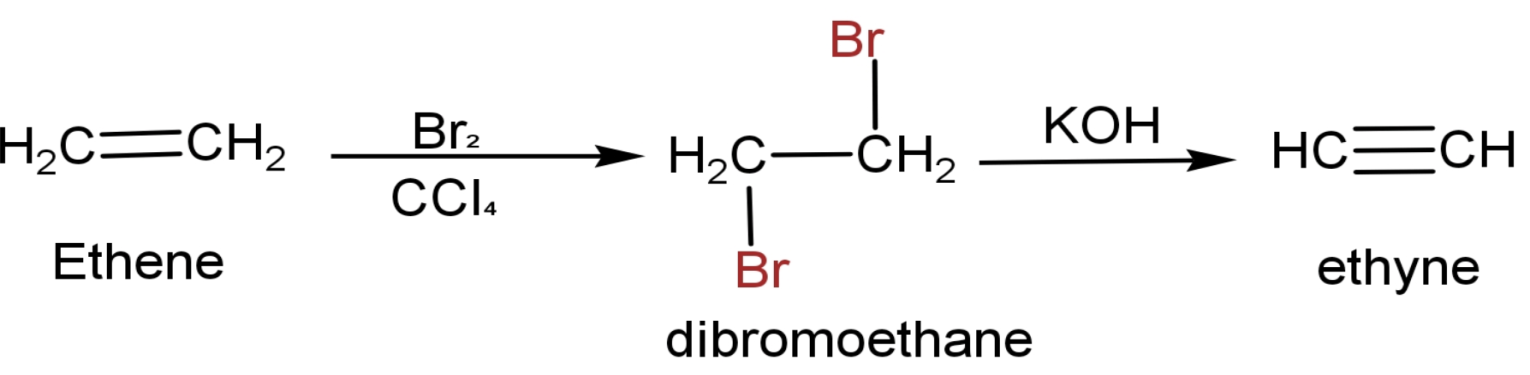
The question can be directly solved as the conversion of ethene to ethyne is given when we talk about preparation of ethynes. Let us see that reaction and the steps to convert ethene to Ethyne are:
(1) Ethene has a chemical formula CH2=CH2.
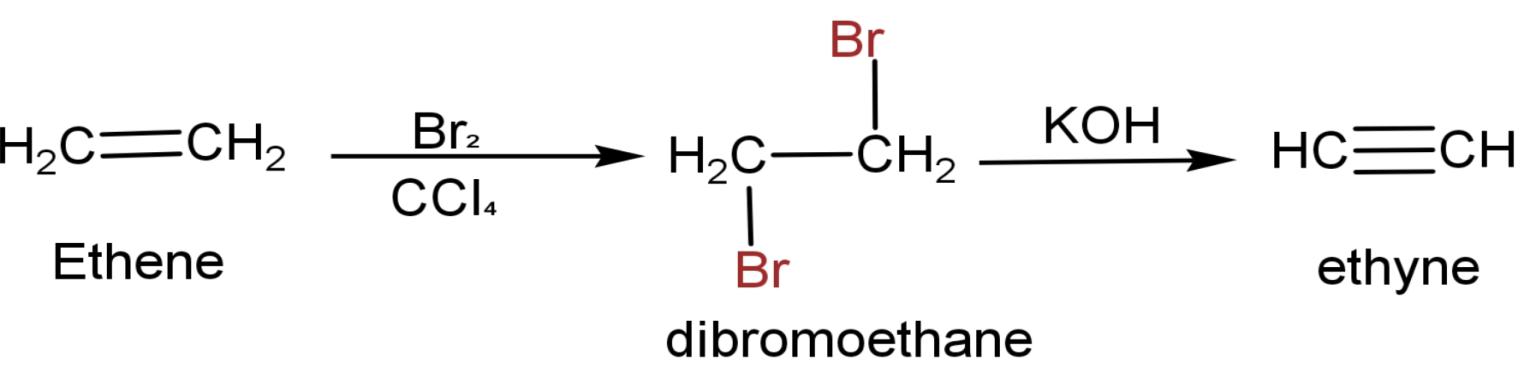
(2) Made it react with Br2 in the non-polar solvent CCl4.Two atoms of Br attaches to both the doubled-bonded carbon and forms dibromoethane (CH2Br-CH2Br). This a type of addition reaction.
(3) Then it is heated in the presence of alcoholic KOH.The end product is ethyne.
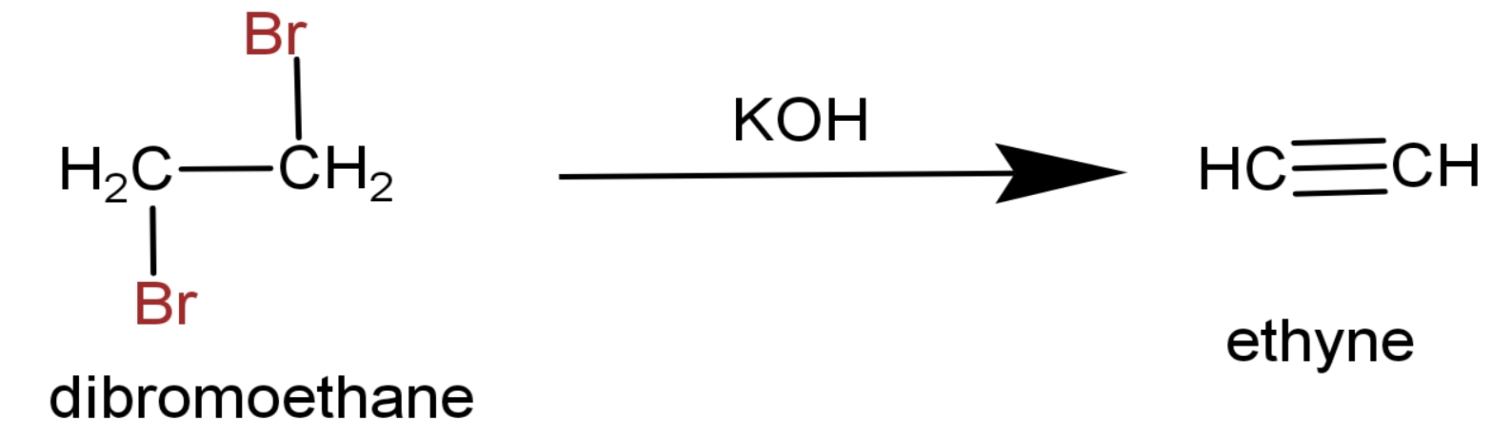
The full reaction is
The correct answer to this question is option ‘d’ which is first bromine water then KOH.
So, the correct answer is “Option D”.
Additional Information: Uses of ethyne:
(1) Calcium carbide was used to produce ethyne used in the lamps for portable and remote applications.
(2) Oxy-acetylene welding equipment is versatile because a torch is preferred for iron or steel welding.
Note: The work each reagent should be known correctly. HereBr2 is used with non-polar solvent like CCl4 that is so that Br2 does not react with polar solvent (like water) to form other products and reaction does not suffer.
