Question
Question: Out of \[S{F_4}\],\[Cl{F_3}\],\[Xe{F_4}\] and \[{H_2}O\] which one is non-planar\[?\]...
Out of SF4,ClF3,XeF4 and H2O which one is non-planar?
Solution
Planarity or nonplanarity is determined by geometry which is further controlled by hybridisation of the central atom in the compound so by knowing the hybridisation we can tell if a compound is planar or not. Hence, the molecule will not be planar if there is an sp3 hybridized carbon (or nitrogen) atom or two sp2hybridized atoms of carbon (or nitrogen) which are separated by an even number of double bonds and no single bonds. Otherwise, its structure allows it to be planar. The polarity of a molecule is basically the measure of its dipole moment. If the dipole is a considerable number (not equal to zero), the molecule is said to be polar.
Complete answer: We have to find the hybridization. To find, first write the Lewis structure to get a rough idea about the structure of the molecule and bonding pattern. Use the valence concept to arrive at this structure. Concentrate on the electron pairs and other atoms linked directly to the concerned atom. Using the Lewis structure, calculate the number of sigma (σ) bonds and the number of lone pairs. Then calculate the steric number. Steric number is the sum of the number of σ-bonds and a number of lone pairs. Now, based on the steric number, it is possible to get the type of hybridization of the atom. Consult the following table.
| Steric number | hybridization | Structure |
|---|---|---|
| 2 | sp | Linear |
| 3 | sp2 | Trigonal planar |
| 4 | sp3 | tetrahedral |
| 5 | sp3d | Trigonal bipyramidal |
| 6 | sp3d2 | octahedral |
| 7 | sp3d3 | Pentagonal bipyramidal |
Number of σ-bonds formed by the atom in a compound is equal to the number of other atoms with which it is directly linked to.
The number of lone pairs on a given atom can be calculated by using the following formula.
Number of lone pairs=2v−b−c
Where v is number of valence electrons in the concerned atom in free state (i.e., before bond formation), b is the number of bonds (including both σand πbonds) formed by the concerned atom and c is charged on the atom.
The Lewis structure of SF4
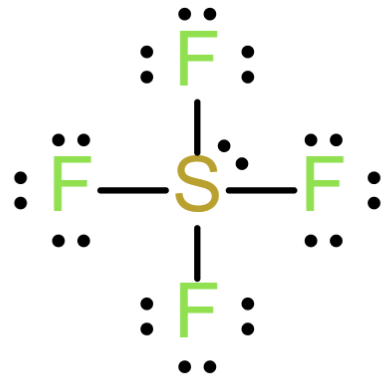
From the above structure, the central atom of oxygen is having 4 sigma bonds and one lone pair. Which implies that the steric number of SF4 is equals to 5 and the geometry\shape is trigonal bipyramidal and the hybridization sp3d.
Hence SF4 is planar.
The Lewis structure of ClF3
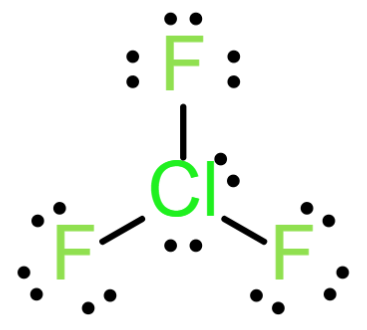
From the above structure, the central atom of Cl is having 3 sigma bonds and 2 lone pairs. Which implies that the steric number of ClF3 is equals to 5 and the geometry is Trigonal bipyramidal and the hybridization sp3d.Due to the T-shaped structure between the central molecule and the 3 bond pair, the molecule is planar.
Hence ClF3 is planar.
The Lewis structure of XeF4 is
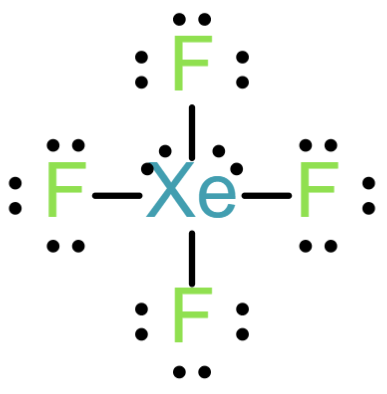
From the above structure, the central atom of Xe is having 4 sigma bonds and 2 lone pairs. Which implies that the steric number of XeF4 is equals to 6 and the geometry is octahedral and the hybridization sp3d2.
Hence XeF4 is nonplanar.
The Lewis structure of H2O is
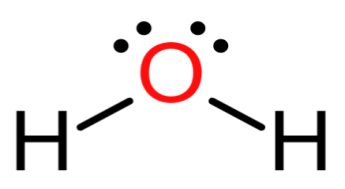
From the above structure, the central atom of oxygen is having 2 sigma bonds and 2 lone pairs. Which implies that the steric number of H2O is equals to 4 and the geometry is tetrahedral and the hybridization sp3. The water molecule arrives at a bent or a V-shape due to the presence of lone pair-lone pair, lone pair-bond pair and bond pair-bond pair repulsions.
Hence H2O is nonplanar.
Hence SF4and ClF3 are planar.
Note:
Note that when the steric number is not equal to the number of σ-bonds, we have to arrive at the shape of the molecule by considering the arrangement of the σ-bonds in space. Though the lone pairs affect the bond angles, their positions are not taken into account while doing so.
If the steric number and the number of σ-bonds are equal, then the structure and shape of the molecule are the same. This case arises when there are no lone pairs on the given central atom.
