Question
Question: Out of (ref. image), which one is more reactive towards \({{S}_{N}}2\) reaction and why? , which one is more reactive towards SN2 reaction and why?
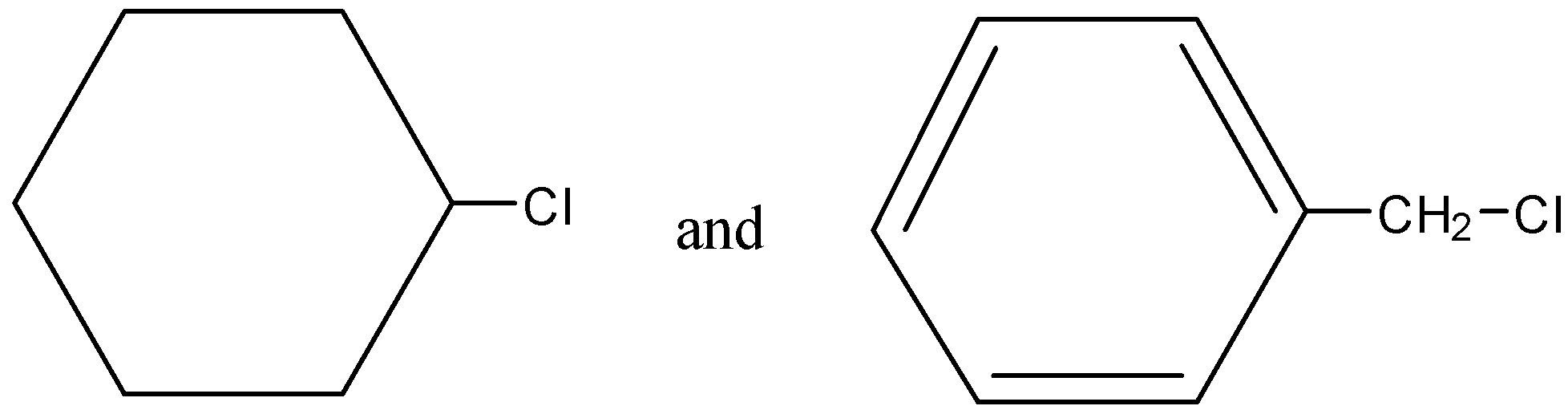
Solution
The order of reactivity of SN1 reaction is : tertiary>secondary>primary , whereas in case of SN2 reaction, the order gets reversed i.e primary>secondary>tertiary.
Complete step-by step solution:
The SN2 reaction could be a kind of reaction mechanism that's common in chemical science. during this mechanism, one bond is broken and one bond is created with synchronisation, i.e., in one step. SN2 may be a quite nucleophilic substitution reaction mechanism, the name touching on the Hughes-Ingold symbol of the mechanism. Since two reacting species are involved within the slow (rate-determining) step, this ends up in the term substitution nucleophilic (bi-molecular) or SN2; the opposite major kind is SN1. Many other more specialized mechanisms describe substitution reactions.
The reaction type is so common that it's other names, e.g. "bimolecular nucleophilic substitution", or, among inorganic chemists, "associative substitution" or "interchange mechanism".
-The reaction most frequently occurs at an aliphatic sp3 carbon center with an electronegative, stable leaving group attached thereto (often denoted X), which is usually a halide atom. The breaking of the C–X bond and also the formation of the new bond (often denoted C–Y or C–Nu) occur simultaneously through a transition state during which a carbon under nucleophilic attack is pentacoordinate, and approximately sp2 hybridised. The nucleophile attacks the carbon at 1800 to the leaving group, since this provides the simplest overlap between the nucleophile's lone pair and therefore the C–X σ∗ antibonding orbital. The leaving group is then pushed off the alternative side and also the product is created with inversion of the tetrahedral geometry at the central atom. If the substrate under nucleophilic attack is chiral, then this often ends up in inversion of configuration (stereochemistry), called a Walden inversion. In our case, as the first compound has chlorine attached to the carbon which has 20 configuration, whereas in the second compound, chlorine is attached to the 10 carbon.
Hence, compound 2 is more reactive towards SN2 reaction mechanism.
NOTE: As we know that during SN2 reaction, attack occurs from the back side. However it may happen that some compounds are sterically hindered, for which SN2 does not take place. In such cases, the major product formed is of SN1 mechanism.
