Question
Question: Out of (ref. image), which is an example of a benzylic halide? , which is an example of a benzylic halide?
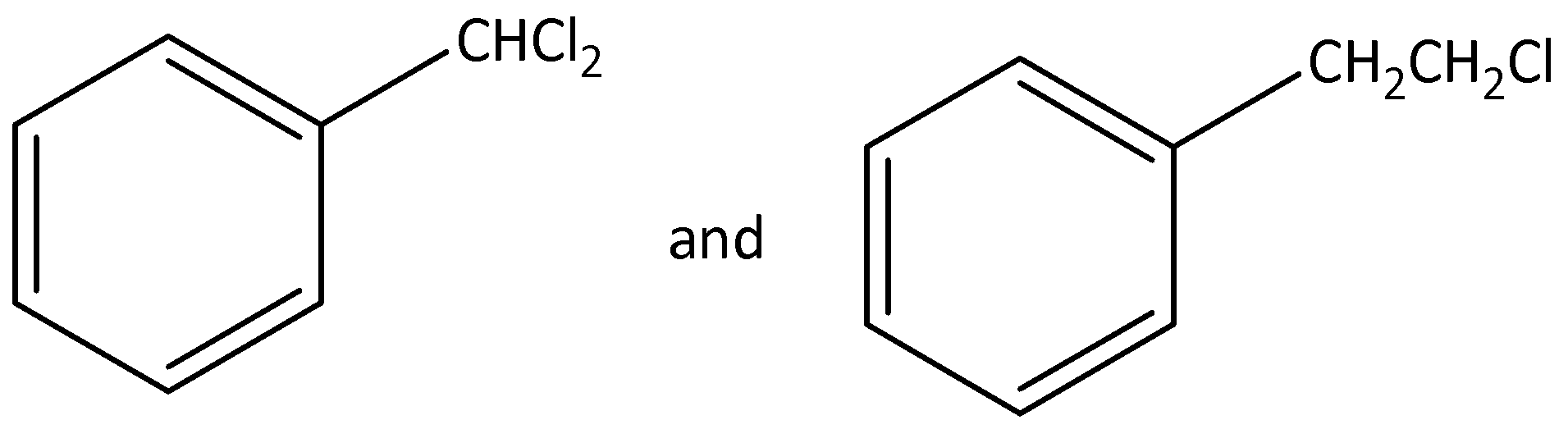
Solution
We have to remember that the benzene is a six membered ring cyclic carbon atom. Benzene is an aromatic compound. The molecular formula of the benzene is C6H6 . In benzene, alternate double bonds are present in six membered rings. Due to this delocalisation of pi electrons in benzene, it forms an aromatic compound.
There are 6 pi electrons in benzene, the one more reason it is considered as an aromatic compound.
Complete step by step answer:
We need to know that the benzylic halide means one more or more halogen atom bonded to a carbon atom, that carbon atom is attached to any carbon atom in the benzene ring.
According to below figure
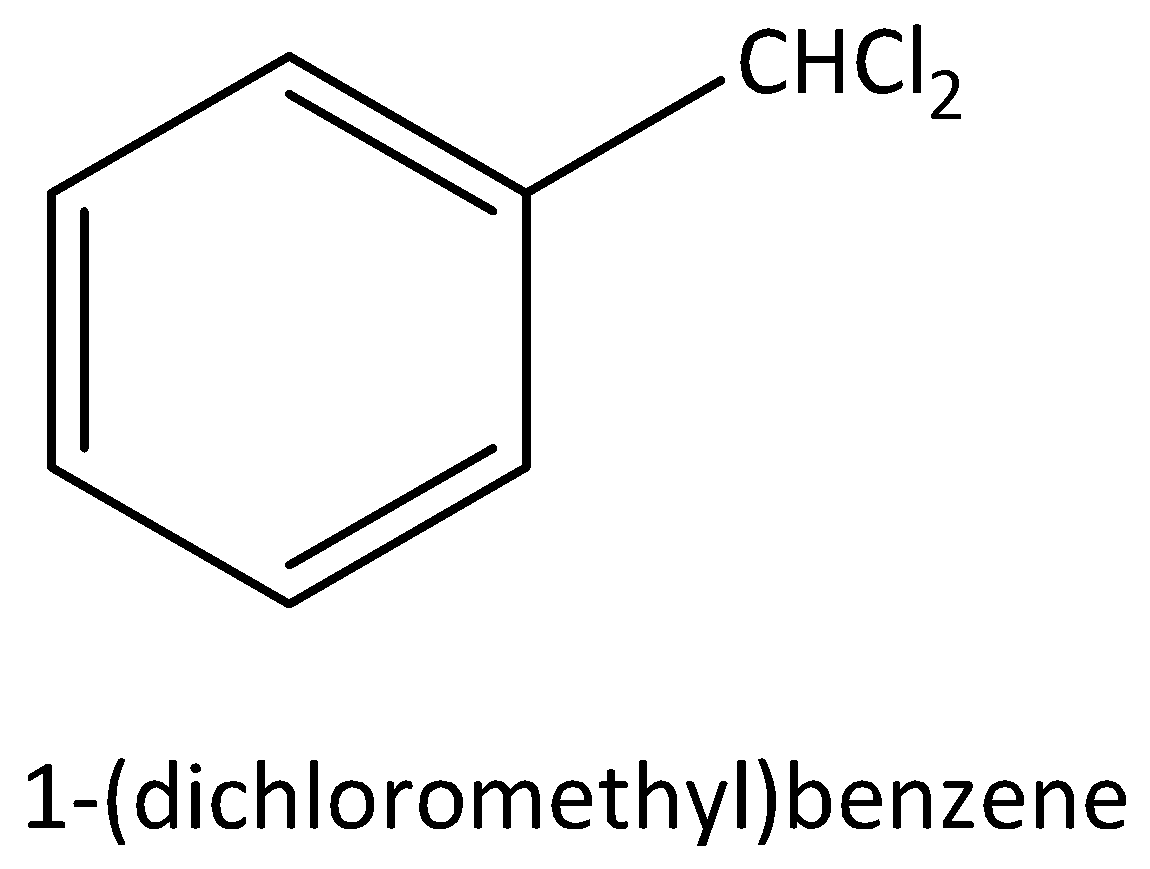
The molecular formula of this figure C7H6Cl2 .
In this molecule two chlorine atoms are bonded to carbon, that carbon is attached to any carbon atom in the benzene ring.
Hence, this molecule is a good example for benzylic halide.
The IUPAC name of this molecule is 1-(dichloromethyl) benzene.
This is an aromatic compound.
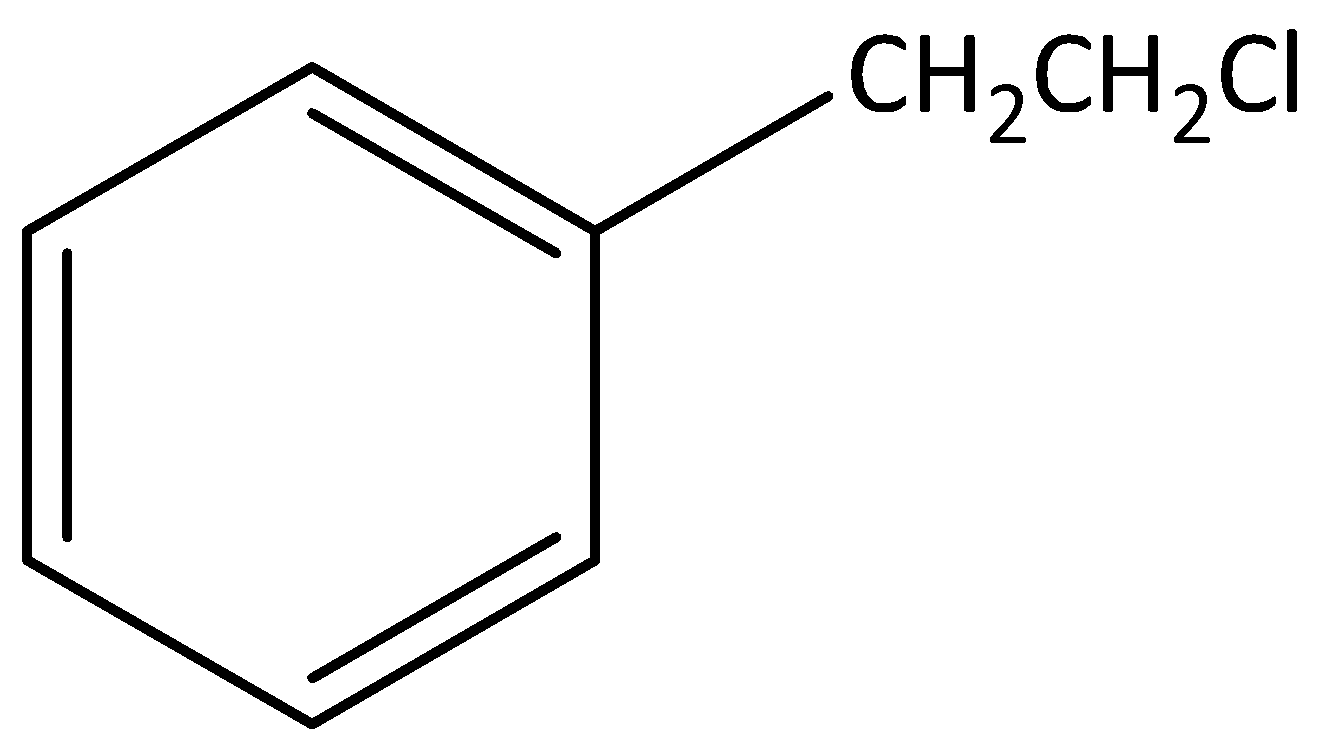
According to below figure
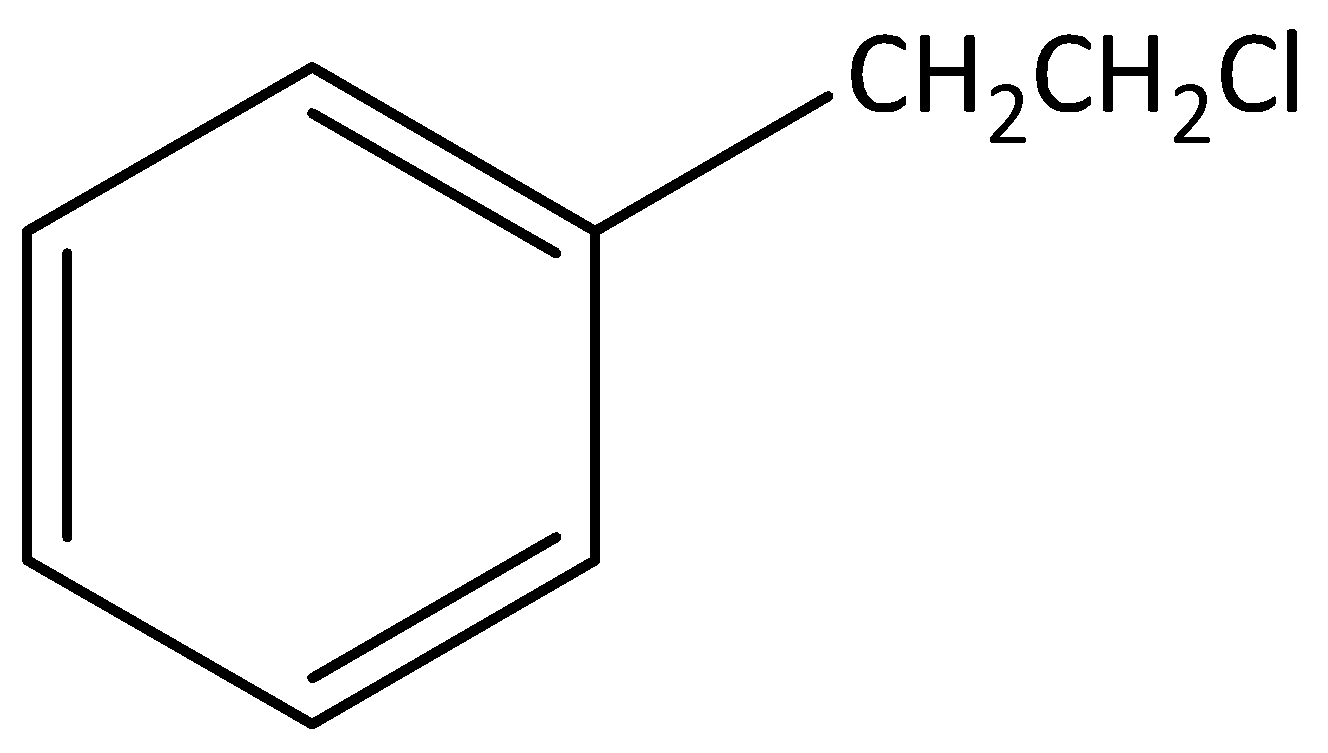
The molecular formula of this figure C8H10Cl1 .
In this molecule one chlorine atom is bonded to carbon, that carbon is not attached to any carbon atom in the benzene ring.
Hence, this molecule is not an example for benzylic halide.
The IUPAC name of this molecule is 1-(2-chloroethyl) benzene.
This is an aromatic compound.
According to above discussion,
Figure one 1-(dichloromethyl) benzene is benzylic halide
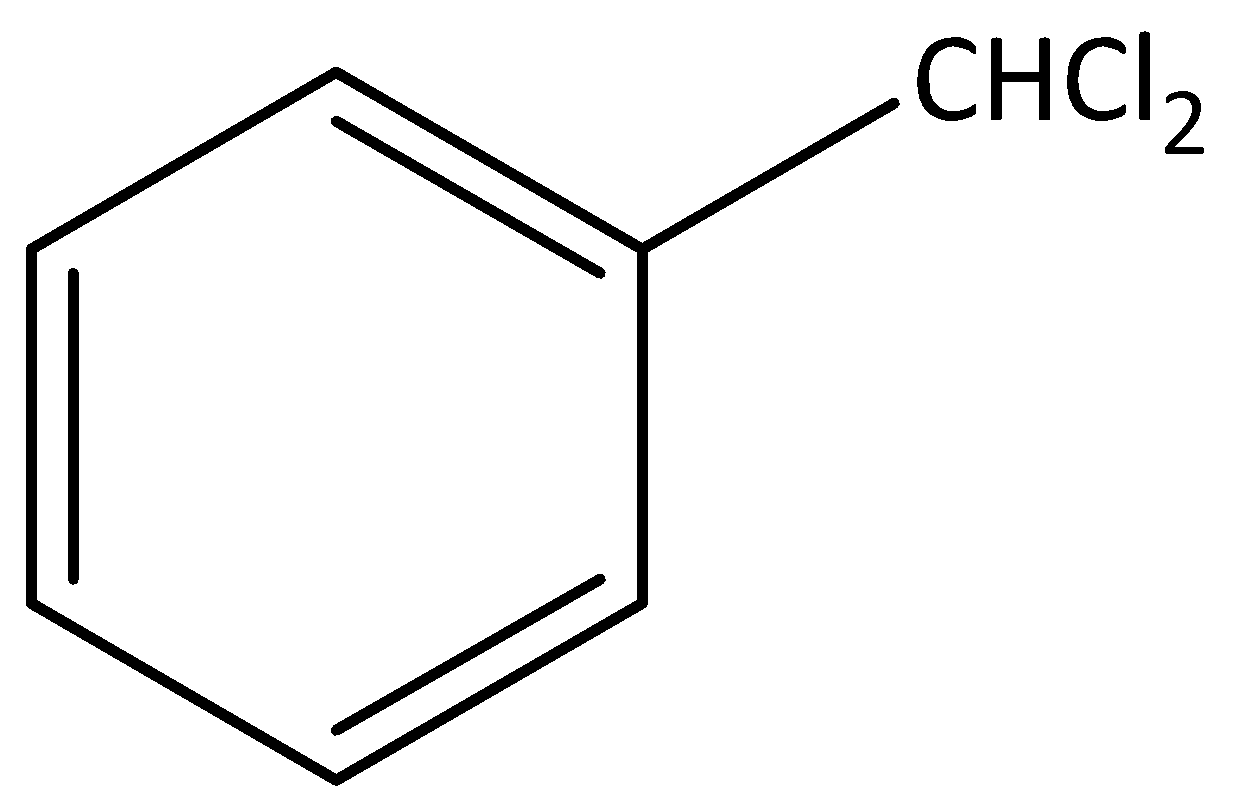
Figure two 1-(2-chloroethyl) benzene is not benzylic halide
Note: Figure one and two are aromatic compounds. Both obey delocalised pi electrons. Resonance structures are also possible in two structures. Both molecules obey (4n+2) rule.
But one is benzylic halide, another is not benzylic halide. Because of the halogen group bonded carbon is different in both molecules.
