Question
Question: Out of \({H_2}{S_2}{O_3},{H_2}{S_4}{O_6},{H_2}S{O_5}{\text{ and }}{H_2}{S_2}{O_8}\) peroxy acids are...
Out of H2S2O3,H2S4O6,H2SO5 and H2S2O8 peroxy acids are:
A. H2S2O3,H2S4O6
B. H2S4O6,H2SO5
C. H2SO5, H2S2O8
D. H2S2O3,H2S2O8
Solution
Peroxy acids are those which contain an acidic group −OOH that is replaced by the −OH group of an oxyacid. Peroxy acids are frequently used as an oxidant and are also known as peracid.
Complete answer: To find the proxy acid out of the options given we will start by analysing the structures of each compound.
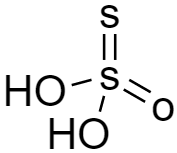
As per the structure of thiosulphuric acid (H2S2O3) is given below, we can see there is no −OOH group hence it is not peroxy acid.
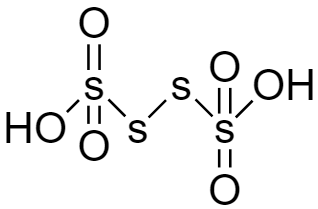
For the structure of Tetrathionic acid (H2S4O6) given below we can see there is no −OOH group hence it is also not a peroxy acid.
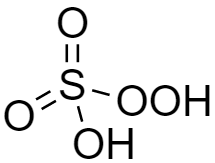
The structure of peroxymonosulfuric acid (H2SO5) is shown below and we can see there is the presence of one −OOH group. Hence it is a peroxy acid. This is also known as Caro's acid.
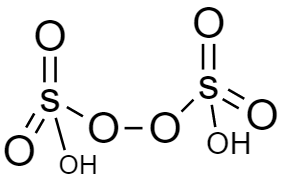
In the structure of peroxodisulfuric acid (H2S2O8), there is peroxy linkage hence this is also peroxy acid. This is also known as marshall’s acid. The structure is given below:
So, the correct answer is “Option C”.
Additional Information: Caro's acid i.e. peroxymonosulfuric acid is probably the most important inorganic peroxy acid, at least in terms of its production scale. It is used for the bleaching of pulp and the detoxification of cyanide in the mining industries. It is produced by treating sulphuric acid with hydrogen peroxide.
Note: Peroxide acid is generally prepared by reaction of the oxy acid with hydrogen peroxide, some of the sulphuric or other strong acids are often used to accelerate the reaction of weak oxy acid. They readily add oxygen to alkenes to give epoxides and are used to convert ketones into ester and amines into the nitro compound, amine oxides, or nitroso compounds.
