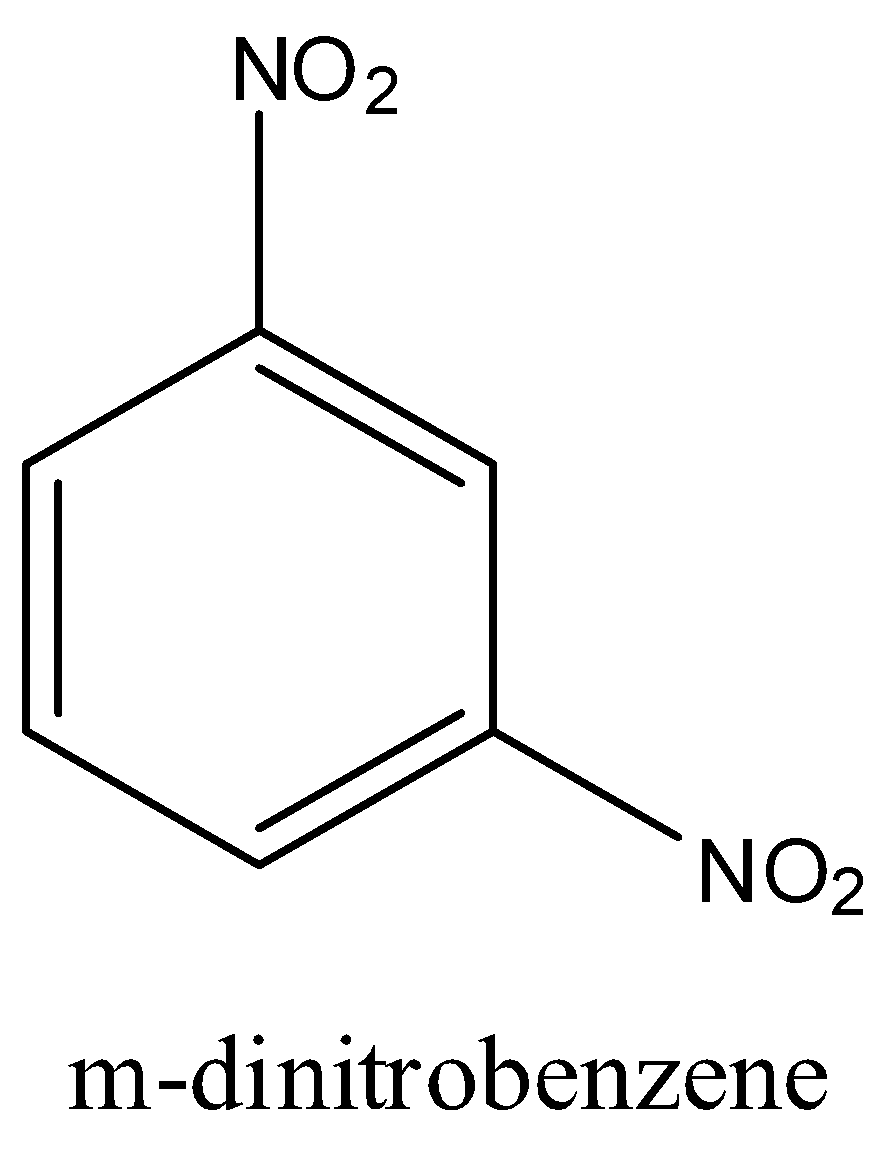
Question
Question: Out of benzene, m-dinitrobenzene, and toluene which will undergo nitration most easily and why....
Out of benzene, m-dinitrobenzene, and toluene which will undergo nitration most easily and why.
Solution
Nitration means addition of nitronium ion (NO2+) and it is a best example for electrophilic addition reaction. Nitronium ion is an electrophile and tries to attach to the molecule where a large number of electrons or high electron density is present.
Complete step by step answer:
In the question it is given that, out of benzene, m-dinitrobenzene, and toluene which will undergo nitration most easily.
First we should know the structures of the given compounds.
The structures of the given compounds are as follows.
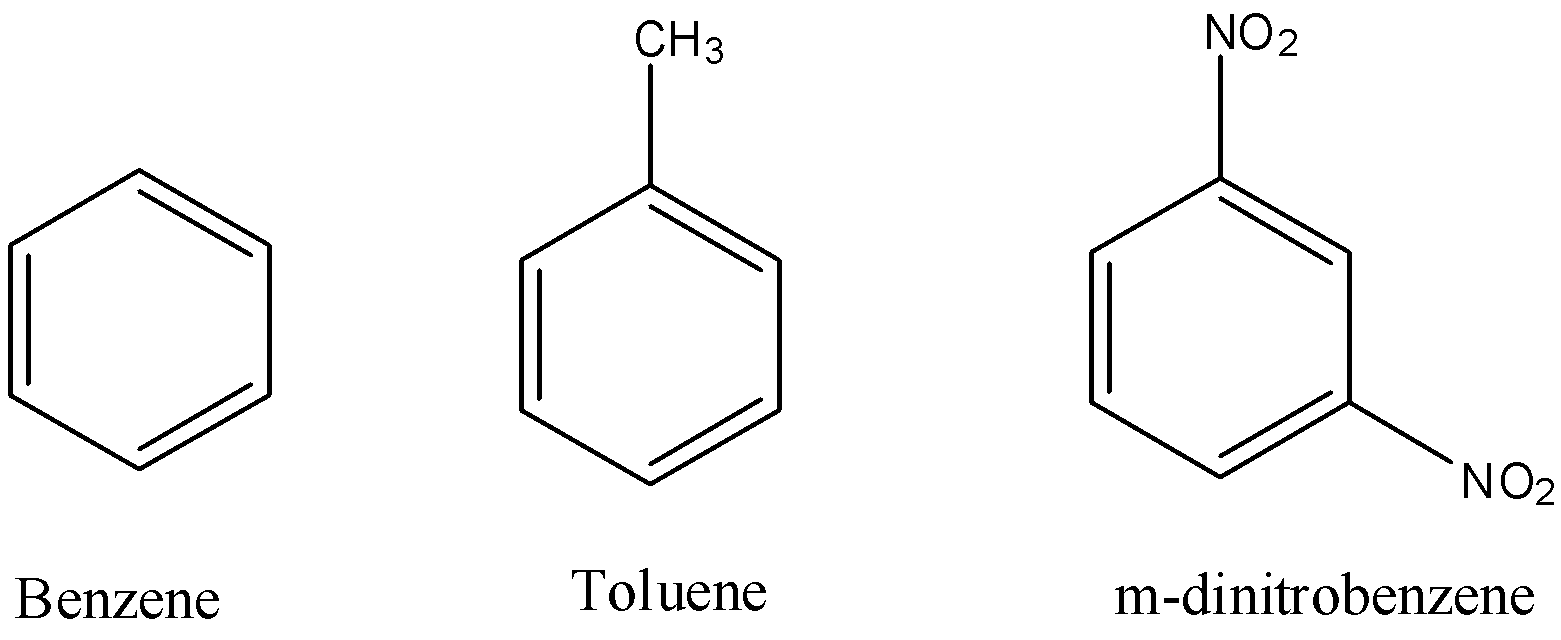
We know that the ease of nitration depends on the presence of electron density on the given compound to form the respective nitrate derivatives.
We know that Benzene is electron rich in nature but all the electrons are in resonance, so benzene itself won't easily donate electrons or won't easily undergo nitration reaction to form nitro derivative.
Toluene is also called methyl-benzene. Methyl group in toluene makes it an electron donating molecule.
So, ease of nitration is very easy in case of toluene when compared to simple benzene.
Coming to m-dinitrobenzene, the presence of two nitro groups makes the molecule as electron deficient. So, nitration in m-dinitrobenzene is very difficult to do.
Hence, the decreasing order of nitration of the given compounds is as follows:
Toluene > Benzene > m-dinitrobenzene
Note: Don’t be confused with nitro benzene and m-dinitrobenzene.
In nitro benzene only one nitro group will be there but in m-dinitrobenzene there are nitro groups in meta position to each other.
The structure of nitro benzene is
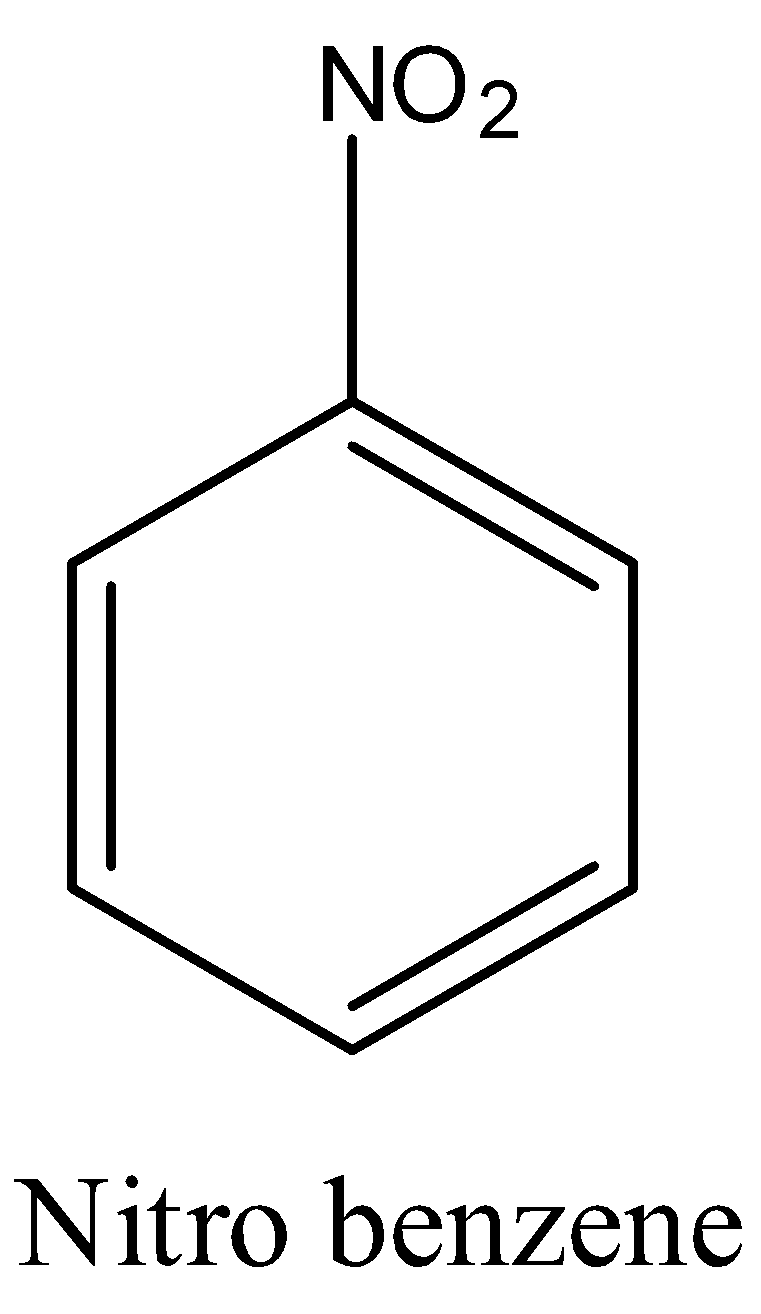
The structure of m-dinitrobenzene is