Question
Chemistry Question on Solutions
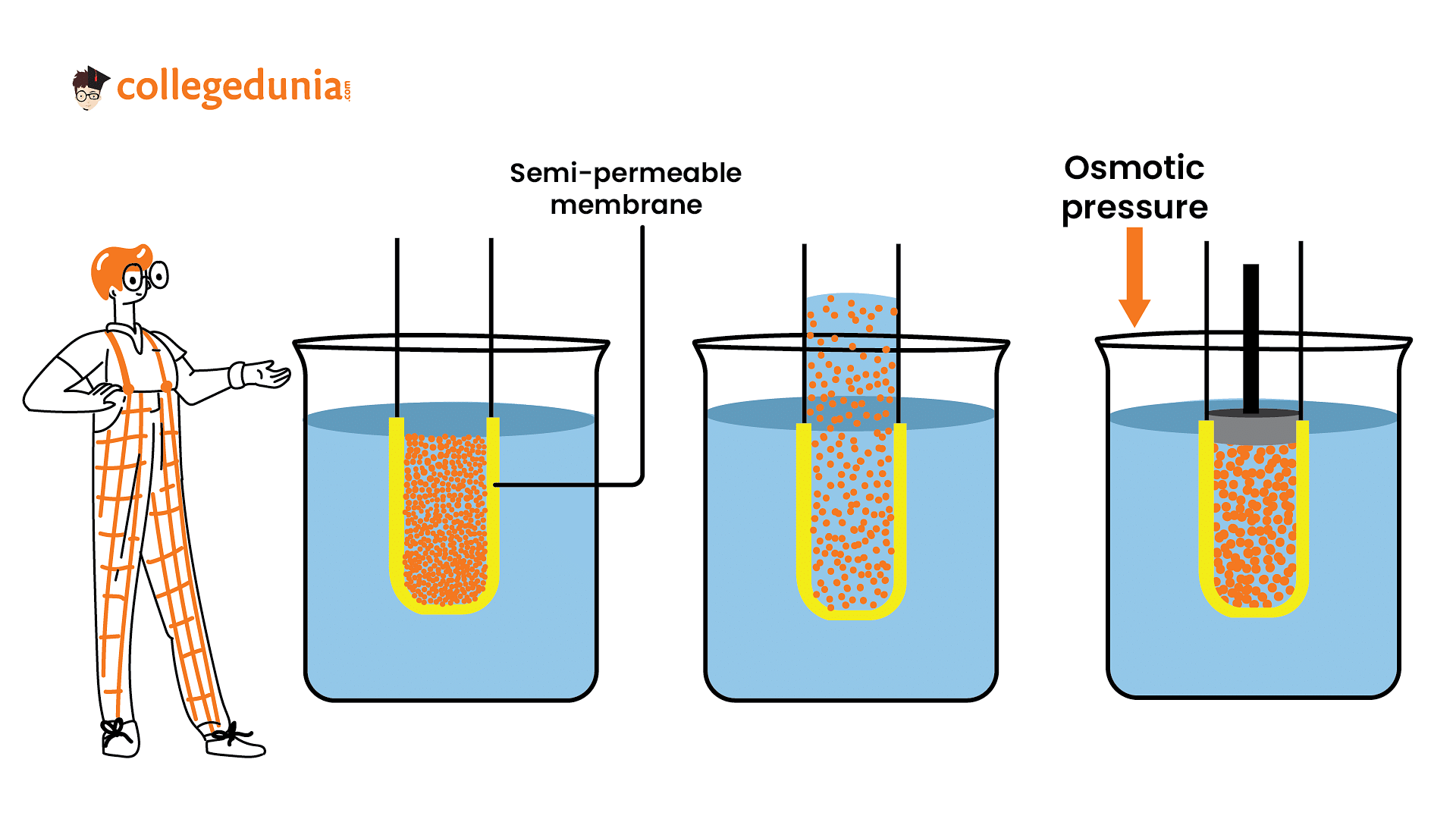
Osmotic pressure can be increased by
A
increasing the temperature of the solution.
B
decreasing the temperature of the solution.
C
increasing the volume of the vessel.
D
diluting the solution.
Answer
increasing the temperature of the solution.
Explanation
Solution
Osmotic pressure is directly proportional to the temperature ∵π=CRT where,
- C= concentration
- R= gas constant
- T= temperature
i.e. π∝T, thus, on increasing the temperature, osmotic pressurealso increases.
Discover More From Chapter:Solutions
