Question
Question: Orthoboric acid \( ({H_3}B{O_3}) \) and metaboric acid \( (HB{O_2}) \) differ in respect of (A) Ba...
Orthoboric acid (H3BO3) and metaboric acid (HBO2) differ in respect of
(A) Basicity
(B) Structure
(C) Melting point
(D) None of the above
Solution
To answer the above question we need to state the properties of both the acid such as structures, melting point and basicity. Also we need to discriminate between both the acids on the basis of these three properties.
Complete step by step answer:
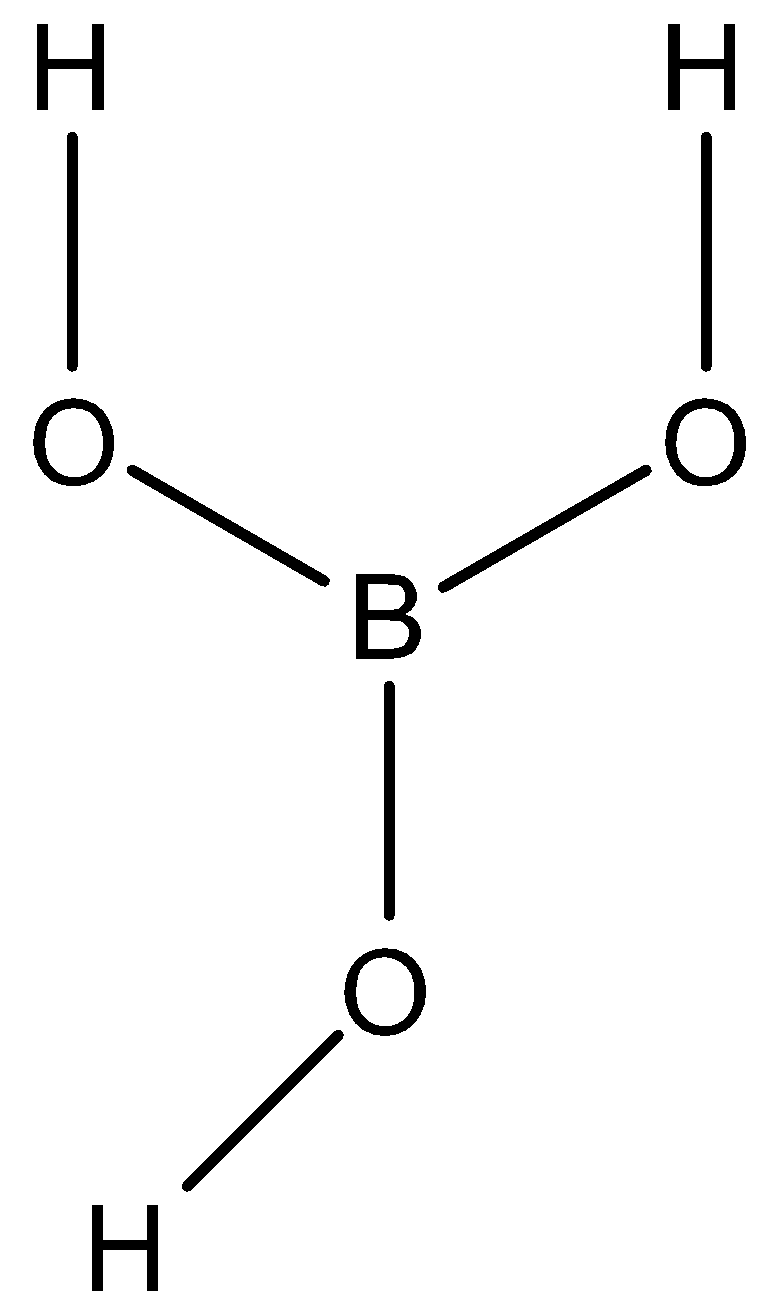
METABORIC ACID (HBO2) : metaboric acid is a common family name for the inorganic compound formed by the dehydration of boric acid. Now we will define metaboric acid on the following three properties:
Basicity: we need pK value to measure the strength of acid on a logarithmic scale. To find out the pK value of an acid we need to use the formula;
pK=log10(1/ka) ;
Where, Ka is the constant used for acid dissociation, now the pKa value for metaboric acid is 9.236.
Structure: metaboric acid is found in two forms, one is the single molecular one and the other one is the chain polymer one.
METABORIC ACID
Melting point: the melting point of metaboric acid is 236oC
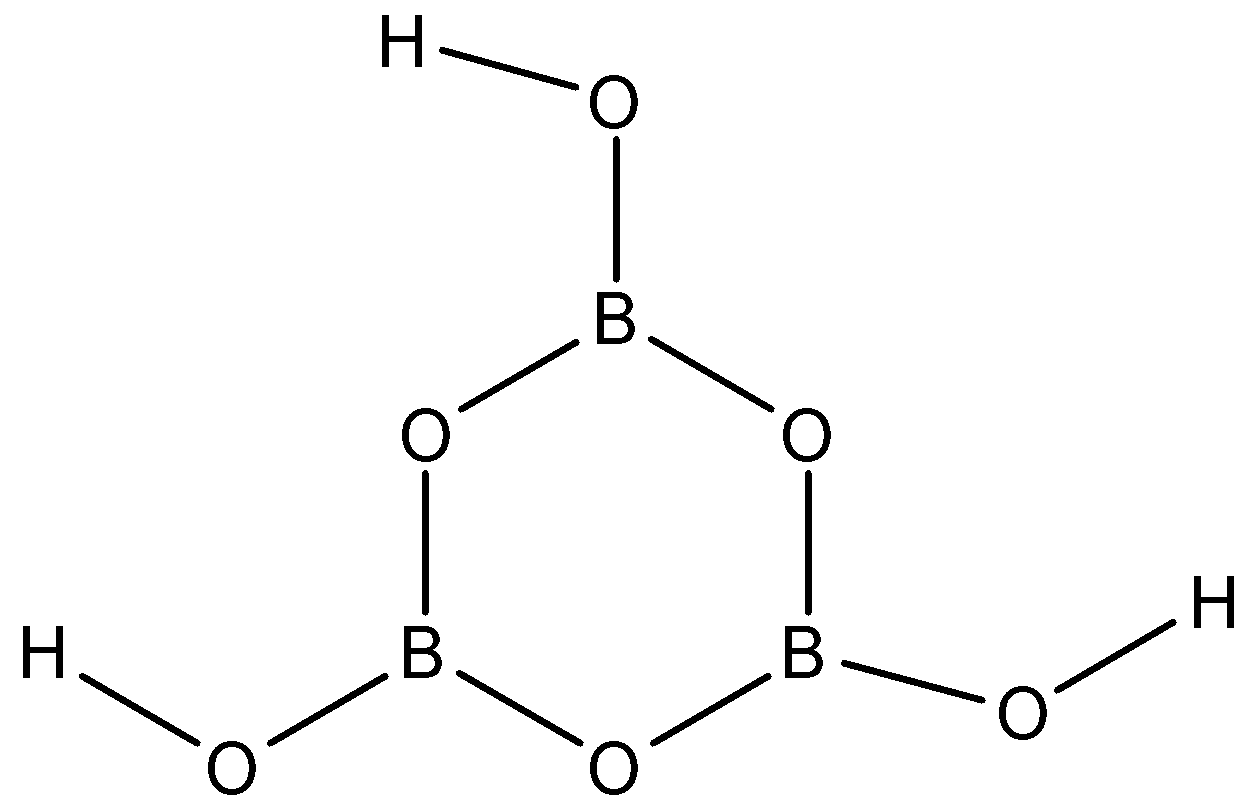
ORTHOBORIC ACID: orthoboric acid is also known as boric acid, hydrogen borate, boracic acid etc. Now we will define orthoboric acid on the following three properties:
-Basicity: the pKa value for orthoboric acid is 9.24, 12.4, 13.3
-Structure: the molecular formula of orthoboric acid is H3BO3
ORTHOBORIC ACID
-Melting point: the melting point of orthoboric acid is 170oC
Now according to the above observation of both the acid we can conclude that orthoboric acid and metaboric acid differs on the basis of basicity, structure and melting point. Therefore all the options A, B, C are correct.
Note:
Both the acids majorly differ in their molecular formula and its structure as metaboric acid is present in two forms molecular and chained and orthoboric acid is only present in molecular form in this case the main difference between both acids.
