Question
Question: Orthoboric acid contains: A.Pyramidal \[B{O_3}^{3 - }\] units B.Linear \[B{O_3}^{3 - }\] units ...
Orthoboric acid contains:
A.Pyramidal BO33− units
B.Linear BO33− units
C.T− Shaped BO33−units
D.Triangular BO33− units
Solution
Orthoboric acid is also known as boric acid. It is a weak boron acid sometimes used as an antiseptic, insecticide or neutron absorber and a precursor to other chemical compounds. The simplest borate anion known as the orthoborate ion BO33− is known.
Complete step by step solution: We can predict which BO33− unit contained in orthoboric acid. The structure of orthoboric acid is,
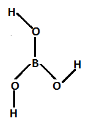
In the structure of orthoboric acid, there are three electron pairs which are three bond pairs. It is sp2 hybridized, the shape of orthoboric acid is triangular planar. The orthoboric acid forms hydrogen bonds with other molecules of orthoboric acid. The orthoboric acid contain Triangular BO33− units
Additional information: Orhtoboric acid has many applications in daily life as well as industry. These are listed below:
Medical use: for minor cut and burn, it can be used as an antiseptic. A very dilute solution of Boric acid can be applied as an eye wash. A dilute solution of boric acid also used as a vaginal douche for the treatment of bacterial vaginosis.
Used as insecticidal: Boric acid can be used as an insecticidal to control ants, cockroaches, fleas and many other insects.
For preserving food: Boric acid is helpful in the preservation of timber against fungal and insect attack.
Used as pH buffer: Boric acid is widely used as pH buffer system in equilibrium with its conjugate base-borate ion (mostly in swimming pools).
For lubrication: Boric acid can be used as lubricant on ceramic or metal surfaces. It can also be used to lubricate carrom boards.
Thus, the correct option is D.
Note: The shape of any molecule can be predicted by Valence shell e− pair repulsion theory, commonly called as VSEPR theory. Here e− pairs refer to the bond pairs and lone pair e−. The geometry of the molecules mainly depends on e− pairs.
