Question
Question: Ortho,para-bromoanisole\(+NaN{{H}_{2}}~+N{{H}_{3~(liq.)}} \) ? How do you predict the product?...
Ortho,para-bromoanisole+NaNH2 +NH3 (liq.) ? How do you predict the product?
Solution
Hint : Benzene mechanism is followed in this reaction. It is formed as benzene then further reaction occurs. Ortho, para-bromoanisole is an electron withdrawing group, it undergoes electrophilic substitution. The halogenation will take place only at the ortho and para positions for anisole and not at the meta position
Complete Step By Step Answer:
We know that these are the reaction conditions for generating benzene intermediates. The reaction of ortho-bromoanisole with potassium amide in liquid ammonia (b. p. −33 ∘C ) is extremely rapid.
Step1: The amide ion attacks the H atom that is ortho to C3 , generating a carbanion.
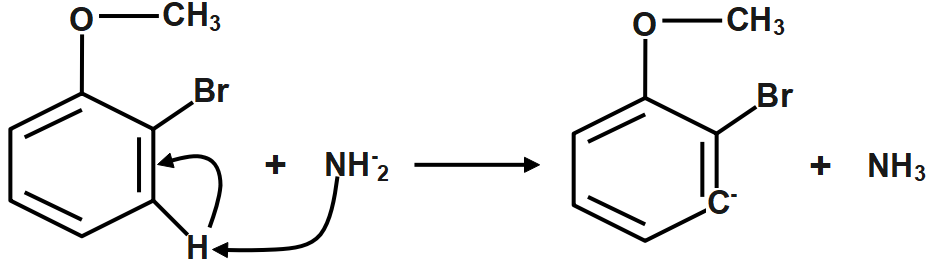
Step2: Loss of Br− to form a benzene intermediate.
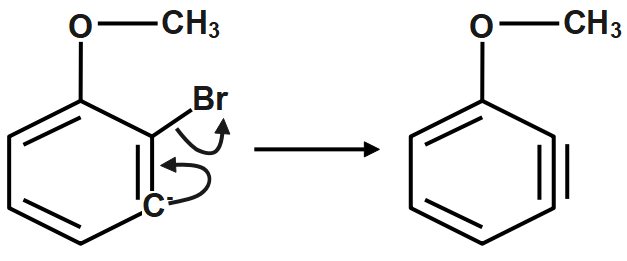
The elimination is by an E2cb pathway.
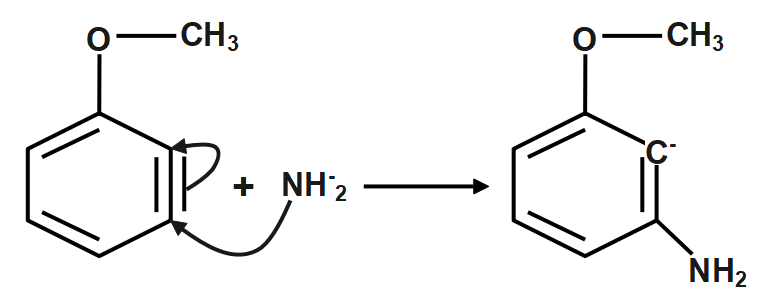
Step3: Addition of NH−2. The strain caused by a triple bond in a benzene ring can be relieved by a nucleophilic addition (AdN) of NH−2. The methoxy group is electron-withdrawing by induction, so the nucleophile will attack C3 to place the carbanion as close as possible to the methoxy group.
Step4: Protonation of the carbanion.
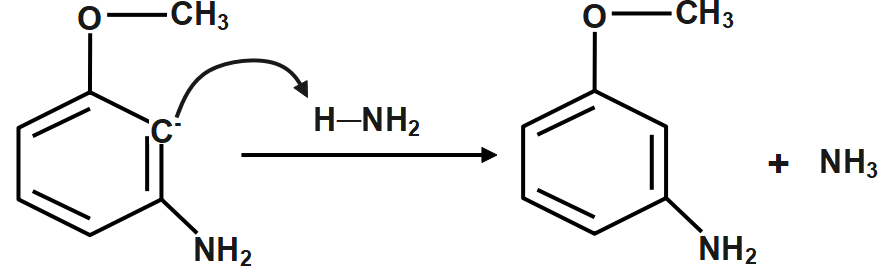
The product is meta-methoxyaniline or para-Bromoanisole
The reaction with para-bromoanisole also follows a benzene mechanism.
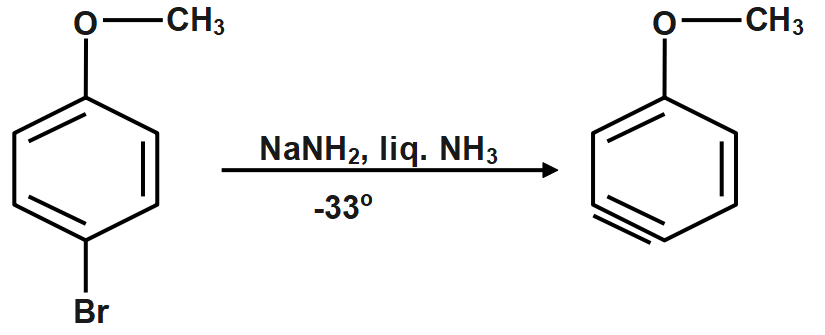
Step5: The nucleophile can attack either end of the triple bond, and the methoxy group is far enough away that its inductive effects are minimal.
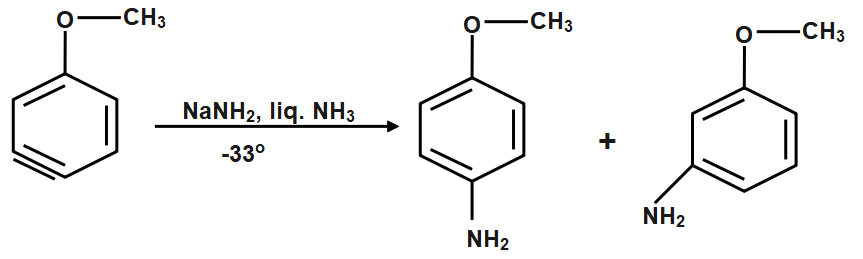
The product is a mixture of para- and meta-methoxyaniline.
Here, we get two products para- and meta-methoxyaniline. Among these two, as we can see para-bromoanisole is the major product and the yield is around 90% for it and the ortho-bromoanisole is the minor product with a very low yield. This is due to the steric hindrance in the ortho position as a bulky methoxy group is attached to the benzene ring.
Note :
As we can see in the above discussion we mentioned that electron withdrawing groups increase the electron density at ortho and para position but the reason behind it can be understood from the resonance hybrid structures of anisole. This is a nucleophilic reaction following the benzene mechanism.
