Question
Question: Ortho and para hydrogen differ: (A) in the number of protons (B) in the molecular mass (C) in ...
Ortho and para hydrogen differ:
(A) in the number of protons
(B) in the molecular mass
(C) in the nature of spins of protons
(D) in the nature of spins of electrons
Solution
A hydrogen molecule is formed when two hydrogen atoms are combined together. A hydrogen atom consists of one proton and one electron.
Complete answer: A proton in an atom of hydrogen is composed of a charge of +1 as well as a spin. And we know that in a hydrogen molecule, we have two hydrogen atoms meaning we have two spins (one spin in each atom of hydrogen). The spin can be in any direction i.e. either clockwise or anti-clockwise. So in case of a hydrogen molecule, we have two possibilities i.e. the two spins can be both aligned (means both clockwise or both anti-clockwise) or in other cases, they can be opposed. Now, let us discuss the case of ortho and para hydrogen.
In Ortho hydrogen, the spins aligned in the same direction whereas in para hydrogen, the spins are aligned in opposite direction as shown in the figure below:
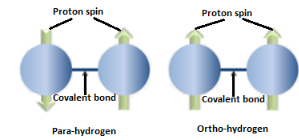
Hence, Ortho and para hydrogen differ in the nature of spins of protons.
Therefore, the correct answer is Option(C) .
Note: The amount of para and ortho hydrogen varies with the temperature. At 0°K, hydrogen is mainly composed of para-hydrogen which is considered to be more stable. At the temperature of air liquefaction, the ratio of ortho and para hydrogen is generally 1 : 1. On the other hand, at room temperature, the ratio of ortho and para hydrogen is 3 : 1.
