Question
Question: Order of stability of carbocation is: (I)  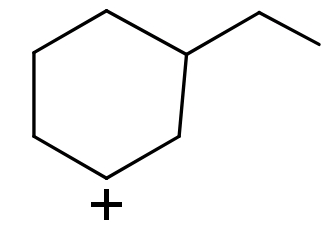
(II) 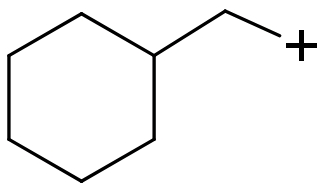
(III) 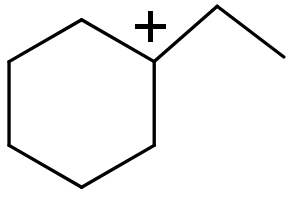
(IV) 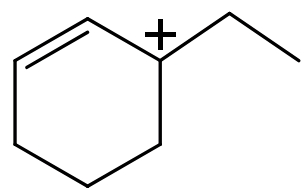
(A) I⟩II⟩III⟩IV
(B) IV⟩III⟩I⟩II
(C) I⟩II⟩III⟩IV
(D) IV⟩III⟩II⟩I
Solution
Carbocation is a reaction intermediate which is formed due to the heterolytic cleavage of C-X bond (where X is more electronegative atom than carbon).
A carbocation is a positively charged and planer in shape (sp2hybridized carbon atom) with 6ein the outermost shell.
Electron donating group (which have positive inductive and mesomeric effect) and hyperconjugation stabilize the carbocation, where electron withdrawing group (have negative inductive and mesomeric effect) destabilise the carbocation
A 3∘ carbocation is more stable than2∘carbocation, and a 2∘carbocation molecule is more stable than 1∘ carbocation molecule.
Resonance is the most stabilizing factor for a conjugate cation and anion because the excess charge on carbon atoms in carbocation and Carbanion can be delocalized by resonance.
Complete answer:
Order of stability of carbocation is 3∘⟩2∘⟩1∘
(I) In this compound carbocation is present on a sp2hybridized 2∘carbon atom.
(II) In this compound carbocation is present on a sp2 hybridized 1∘ carbon atom. So, this carbocation will be least stable.
(III) In this this compound carbocation is present on a sp2 hybridized 3∘ carbon atom.
(IV) In this compound carbocation is present on a sp2 hybridized 3∘ carbon atom and this carbocation is also stabilised by the resonance. Hence this carbocation has the highest stability.
Hence the order of stability is IV⟩III⟩I⟩II
So, option (B) will be the correct option.
Note:
More the number of carbons attached to the carbon carrying positive charge, more the stability of carbocation because bonding electrons attached to the carbocation helps in alleviating the positive charge by overlapping the unoccupied p-orbital of the carbocation.
