Question
Question: Orbital \[{{d}_{{{x}^{2}}-{{y}^{2}}}}\] is involved in which of the following hybridizations? This...
Orbital dx2−y2 is involved in which of the following hybridizations?
This question has multiple correct options.
A.sp3d (square pyramidal)
B.dsp2
C.sp3d2
D.sp3d3
Solution
In organic chemistry, hybridisation is used to explain the covalent bonds in organic molecules. It is defined as the intermixing of atomic orbital of different shape and has the same energy to give the same number of hybrid orbital of the same energy, orientation and shape so that there will be minimum repulsion between the hybrid orbitals.
Complete step-by-step answer: dx2−y2orbital lies in the xy plane in which the lobes are directed along x and y axis. The lobes of orbitals px and py are along the x and y axis.
Let us discuss the above options:
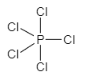
A.The shape for sp3dhybridization is trigonal bipyramidal. It contains one s orbital with three p orbitals that are px,py and pz and one d orbital that is dz2 . The example of sp3dhybridization is PCl5 . Therefore, this option is incorrect.
Let us see the structure:
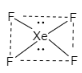
B.As we have discussed that dx2−y2orbital lies in the xy plane in which the lobes are directed along x and y axis. The lobes of orbitals px and py are along the x and y axis. In dsp2 , it has square planar geometry that means the four hybridized orbital lies in xy plane. dx2−y2 orbital is used in dsp2 geometry having square planar shape. This hybridization involves s,px and py orbital. The example of dsp2geometry is XeF4 geometry. Therefore, this option is correct.
Let us see the structure:
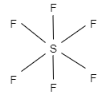
C.The shape of sp3d2 hybridization is octahedral. It contains s,p and d orbitals which undergo mixing to form six identical sp3d2orbitals. They are inclined at an angle of 90∘ to one another. Here, the d orbital is dx2−y2 and dz2 . Therefore, this option is correct. The example of sp3d2is SF6 .
Let us see the structure:
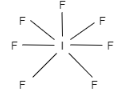
D.The shape of sp3d3 hybridization is pentagonal bipyramidal. It contains ones orbital with p orbitals that are three px,py and pz and three d orbitals. The d orbitals involved are dx2−y2,dz2,dxy . Therefore, this option is correct. The example is Iodine heptafluoride.
Let us see the structure:
Note: It is to note that the type of hybridisation tells us the geometry of a molecule. The hybridised orbitals have equal energy and shape. In hybridisation, atomic orbitals are combined to form a new atomic orbital. The number of hybrid orbitals is equal to the number of orbitals in the hybridisation.
