Question
Question: Orange colour of \( C{{r}_{2}}{{O}_{7}}^{2-} \) ion changes to yellow when treated with an alkali. W...
Orange colour of Cr2O72− ion changes to yellow when treated with an alkali. Why?
Solution
The chromate anion, CrO42− , is found in chromate salts. The dichromate anion, Cr2O72− , is found in dichromate salts. They are relatively strong oxidising agents that are chromium oxyanions in the 6+ oxidation state. The chromate and dichromate ions can interconvert in an aqueous solution.
Complete answer:
An alkali is a basic ionic salt of an alkali metal or an alkaline earth element in chemistry. A base that dissolves in water is also known as an alkali. The pH of a soluble base solution is more than 7.0. Because alkalis were the first bases found to obey the Arrhenius definition of a base, and they are still among the most frequent bases, this broad use of the term is likely to have arisen.
The chromate and dichromate anions are in a chemical equilibrium in aqueous solution.
2 !! !! CrO42-+ 2 !! !! H+ !! !! ⇌ !! !! Cr2O72- !! !! + H2O
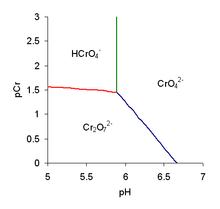
The position of the equilibrium is dependent on both pH and the analytical concentration of chromium, as shown in the predominant diagram. In alkaline solutions, the chromate ion is the major species, but in acidic solutions, dichromate can become the dominating ion.
When Cr2O72- ion is exposed to an alkali, the orange colour of the ion changes to yellow due to the creation of the yellow chromate ion.
Cr2O72−(Orange)+2OH−→2CrO42−(Yellow)+H2O
Because there is no change in hydrogen ion concentration in this equilibrium, it should be pH independent.
The chromate and dichromate ions are moderately powerful oxidizers. A chromium atom is commonly reduced to oxidation state +3 by adding three electrons. The aquated Cr3+ ion is generated in acid solution.
Chromium(III) hydroxide is formed in an alkaline solution. In alkaline solution, chromates are a weaker oxidising agent than in acid solution, according to the redox potential.
Note:
Chrome plating uses chromates and dichromates to protect metals from corrosion while also improving paint adhesion. Heavy metal chromate and dichromate salts, lanthanides, and alkaline earth metals are employed as pigments because they are only very little soluble in water. Chrome yellow, a lead-containing pigment, was used for a long time before environmental rules made it illegal.
