Question
Question: The specific rotation of optically pure 2-butanol has a specific rotation of +13.52 degree. A synthe...
The specific rotation of optically pure 2-butanol has a specific rotation of +13.52 degree. A synthesized and purified sample of 2-butanol has the observed specific rotation of +6.76 degrees. The correct statement based on this observation is
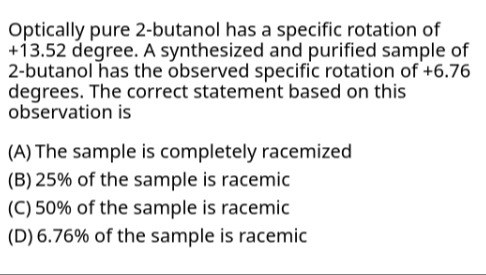
The sample is completely racemized
25% of the sample is racemic
50% of the sample is racemic
6.76% of the sample is racemic
50% of the sample is racemic
Solution
The enantiomeric excess (ee) is calculated as the ratio of the observed specific rotation to the specific rotation of the pure enantiomer, multiplied by 100%. For the given sample,
ee = (+6.76 / +13.52) * 100% = 50%.
An enantiomeric excess of 50% means that 50% of the sample is the excess enantiomer, and the remaining (100% - 50%) = 50% of the sample is a racemic mixture.
