Question
Question: Optically active compound: (This question has multiple correct answers) (a)- Rotates plane-polariz...
Optically active compound: (This question has multiple correct answers)
(a)- Rotates plane-polarized light
(b)- Are asymmetric
(c)- Has enantiomers
(d)- Are symmetric
Solution
If the carbon atom has all the attached groups different then it is an asymmetric molecule, and if the carbon atom doesn't have all the groups attached different then it symmetric carbon. The carbon atom which has all the groups different shows optical activity.
Complete step by step answer:
The optical activity of the compound is the ability of the compound to rotate the plane of polarized light. And optically active compounds are those which rotate the plane of polarized light.
Ordinary light consists of electromagnetic waves of different wavelengths, but monochromatic light has only one wavelength. And plane-polarized light is a monochromatic light.
So those compounds which have a carbon atom on which the attached groups are different can rotate the plane of polarized light. This means that the compound must be asymmetric. If the compound is symmetric then it will not show optical activity or it is an optically inactive compound. The carbon which has all 4 groups attached differently is termed as chiral carbon atom in that molecule.
So, the optically active compounds which are non-superimposable mirror images of each other are called enantiomers and the phenomenon is called enantiomerism.
An example of an optically active compound is bromochloroiodomethane. It is asymmetric and has a non-superimposable mirror image.
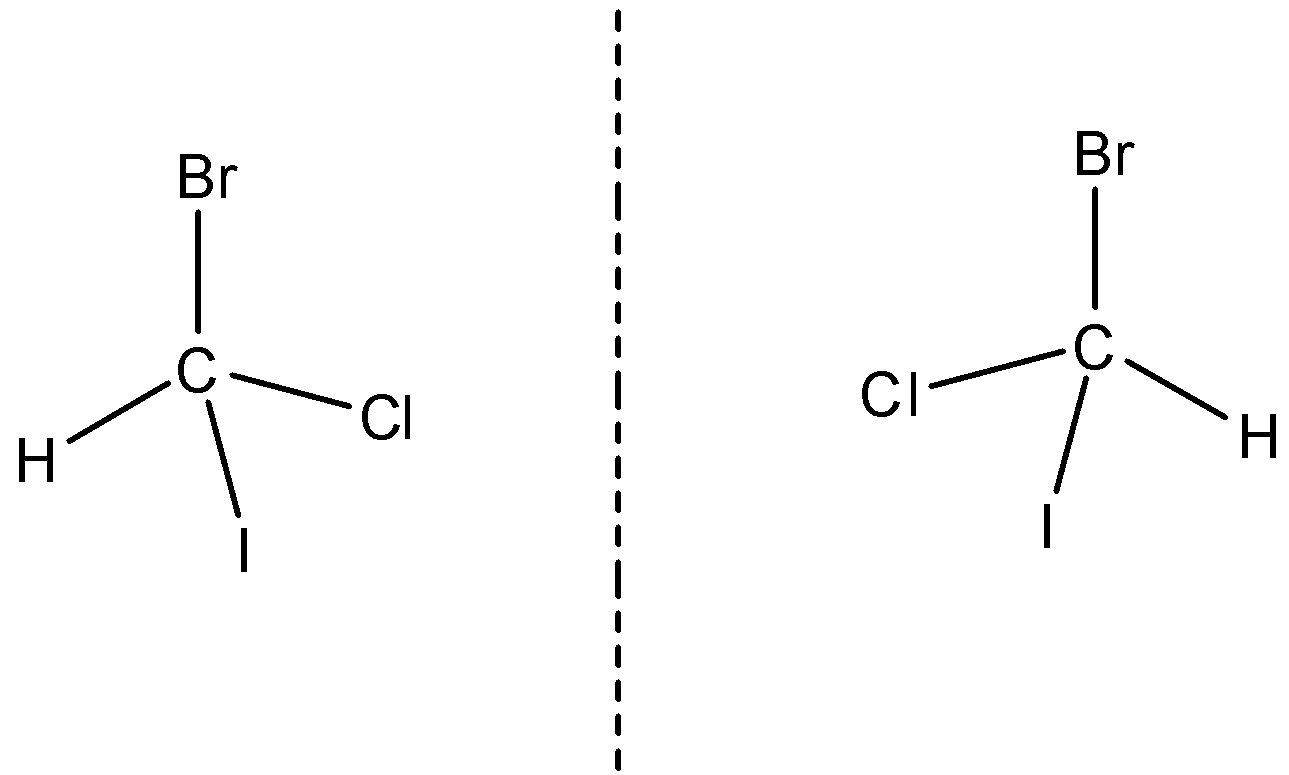
So, the correct answer is “Option A,B and C”.
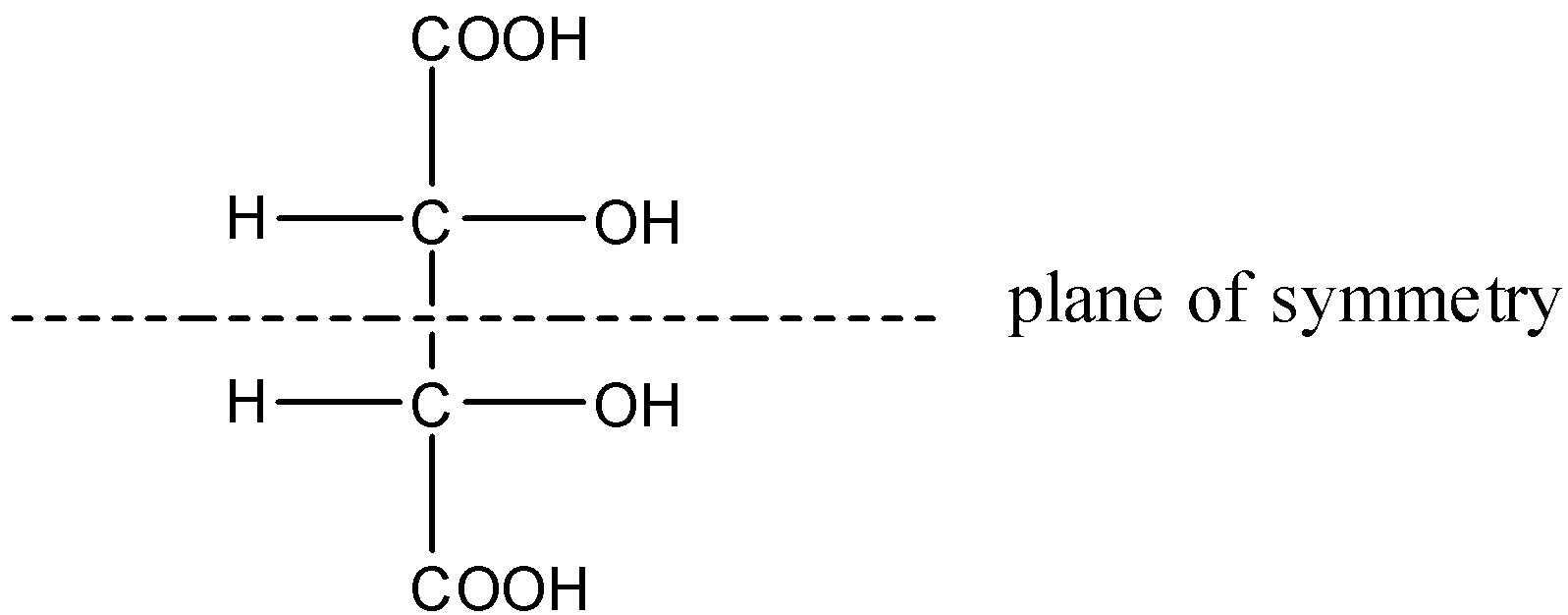
Note: Some compounds have chiral carbon atoms but even though they do not show optical activity, this is due to the plane of symmetry in the overall molecule. These molecules are called Meso compounds.