Question
Question: Only two isomeric monochloro derivatives are possible for(excluding stereo): A. n-butane B. 2,2 ...
Only two isomeric monochloro derivatives are possible for(excluding stereo):
A. n-butane
B. 2,2 dimethylpentane
C. benzene
D. neopentane
Solution
The compound that gives two monochloro derivatives in presence of sunlight is a straight chain compound. It is also used as a fuel as gas and in perfumes too.
Complete answer:
The process of inserting a single chlorine atom in an organic compound(alkane) by the application of sunlight is called monochlorination. Monochlorination is a photochemical reaction and it occurs by free radical generation. The chlorine radical replaces the place of hydrogen in the alkane. The more hydrogen a compound has, for substitution, the more variety of products are formed by monochlorination. That is why it is not biased towards one particular hydrogen. As it is a photochemical reaction, UV light is also required, which is contained in the sun's rays.
Now, let us come to our question. We have n-butane which is a 5 membered straight chain compound. Chlorine can attack and substitute 2 hydrogens:
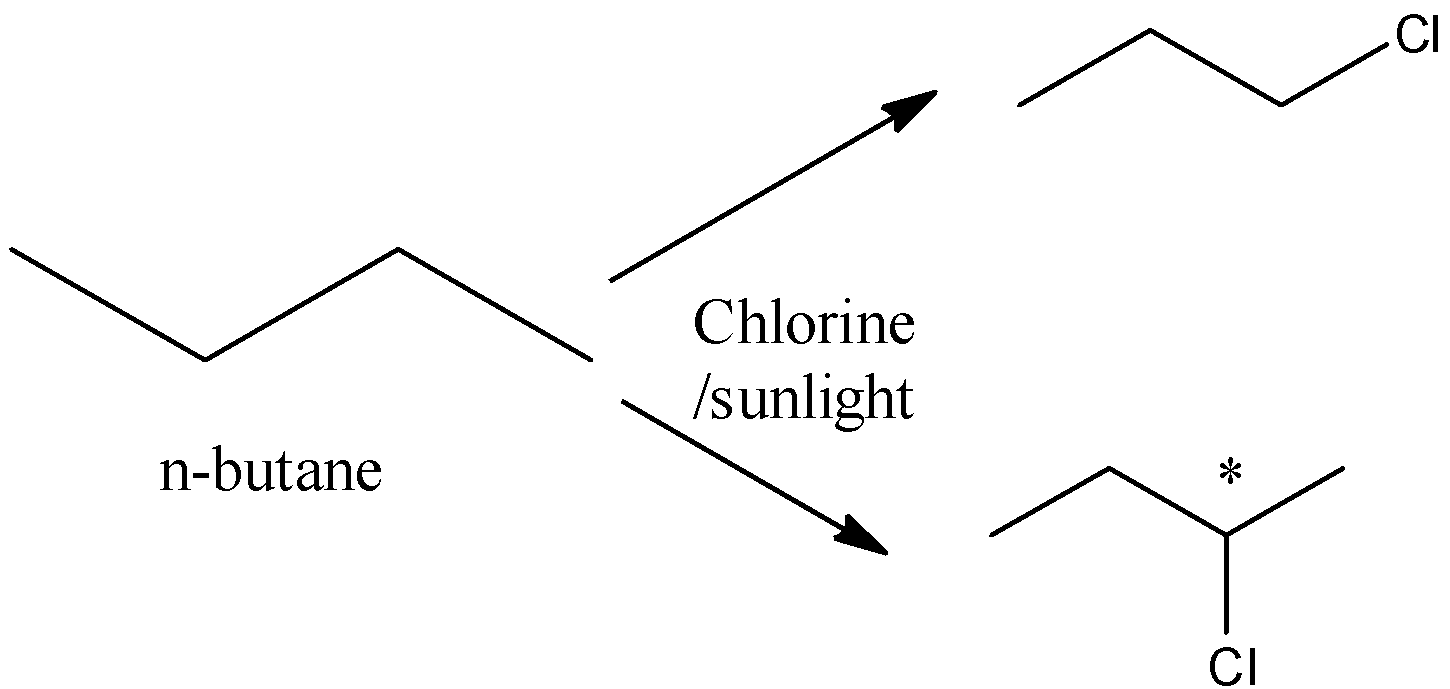
So, we obtain 2 products where in one product, chlorine attacks the terminal hydrogen and the second carbon’s hydrogen respectively. In all the other compounds, there are more hydrogen available for substitution, so the number of products will be more, too. For example, neopentane, although it has a straight chain, has one hydrogen extra than n-butane available for substitution, so it will have 2+1=3 products. The products we obtain in here are 1-chloro butane and 2 chloro butane respectively.
Note:
Remember that in the second product i.e 2-chloro butane, there is a chiral centre present. A chiral centre is a carbon that has different groups attached in different sides. The compound is optically active and has 2 stereoisomers. So, if the question had asked for monochlorination products including stereoisomers, there would have been 3 products of n-butane.
