Question
Question: Only two isomeric monochloro derivatives are possible for (excluding stereo): A.n-butane B.2,2-d...
Only two isomeric monochloro derivatives are possible for (excluding stereo):
A.n-butane
B.2,2-dimethylpentane
C.Benzene
D.neopentane
Solution
Isomers are molecules with each of two or more compounds with an equivalent formula but a special arrangement of atoms within the molecule and different properties. For each hydrogen atom present, a chlorine atom is often replaced. But not all of them would require substitution. Look out for the symmetry within the molecules apart from stereoisomers.
Complete step by step answer:
First, allow us to understand what we mean by monochloro derivatives. When a hydrocarbon is given we will substitute any of its hydrogen atoms with a chlorine atom. A monochloro derivative is made when only a specific carbon atom within the whole molecule has one chlorine atom. Say for instance we are given hydrocarbon ethane. We all know that it’s two carbon atoms. So to form a monochloro derivative out of it, we substitute anyone hydrogen atom attached to the anyone carbon atom. It’s as shown below:
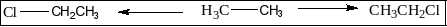
As it is clearly visible, both the monochloro derivatives are an equivalent compound due to the line of symmetry present within the ethane molecule. So within the compounds given above, we’ve to require care of this factor and only map out those monochloro derivatives of a specific molecule that are different from one another structurally.
Let us list out the structure of all the compounds given within a serial wise manner:
A) n-butane : it has the two different isomeric structures. So, it shows only two monochloro derivatives. Therefore, the option A is correct.
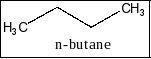
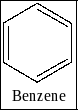
B) Benzene: There is no substituent in benzene structure so it has only one structure. Therefore the option B is incorrect. The structure of benzene is given below as,
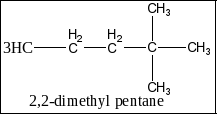
C) 2,2 dimethyl pentane: In 2,2-dimethyl pentane, the two methyl groups are present in the second carbon so possible structure for 2,2-dimethyl pentane. Therefore, the option C is incorrect. The structure of 2,2-dimethyl pentane is given below as,
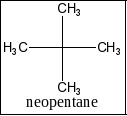
D) Neopentane: This is also called as 2,2-dimethylpropane three isomeric structure and it wouldn’t be possible for monochloro derivatives. Therefore, this option is wrong.

Thus option A is correct.
Note:
We must remember that the n-butane can have two isomeric monochloro derivatives (at positions 1 and 2). Benzene, neopentane, 2,2 dimethylpentane will have one only structure. This was a simple case as the molecules only contained one such line but things could get complicated if we have more of them.
