Question
Question: $Cr_2O_7^{2-} \longrightarrow Cr^{+3}$ [eq". was given] Eq. mass of $Cr = ?$ ...
Cr2O72−⟶Cr+3
[eq". was given] Eq. mass of Cr=?
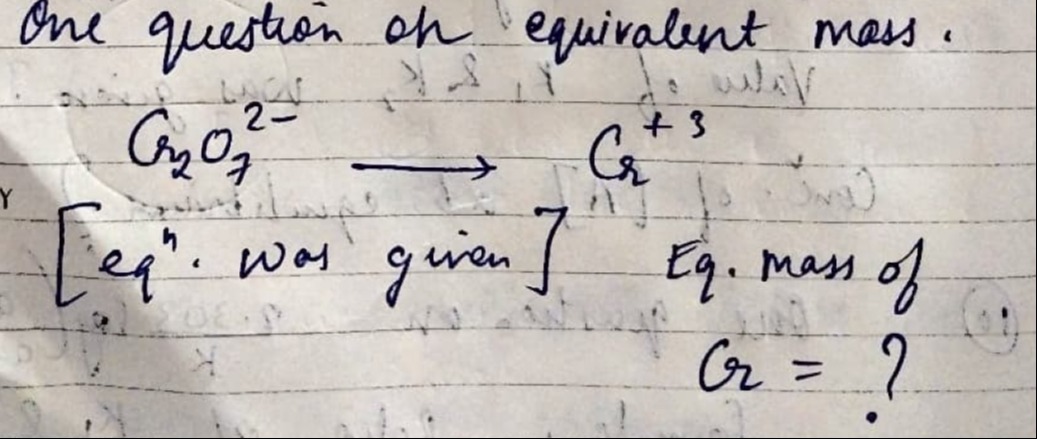
M/3
Solution
To determine the equivalent mass of Cr in the given reaction Cr2O72−⟶Cr+3, we need to find the change in the oxidation state of Chromium.
- Determine the oxidation state of Cr in Cr2O72−:
Let the oxidation state of Cr be x. The oxidation state of oxygen is -2.
For the Cr2O72− ion, the sum of oxidation states must equal the charge of the ion (-2).
2(x)+7(−2)=−2
2x−14=−2
2x=14−2
2x=12
x=+6
So, the oxidation state of Cr in Cr2O72− is +6.
- Determine the oxidation state of Cr in Cr+3:
The oxidation state of Cr in Cr+3 is directly given as +3.
- Calculate the change in oxidation state per Cr atom:
The change in oxidation state for one Cr atom is the absolute difference between its initial and final oxidation states:
Change = ∣(+6)−(+3)∣=3
This change represents the number of electrons gained by one Cr atom. In a redox reaction, this value corresponds to the n-factor (or valency factor) for the element.
- Calculate the equivalent mass of Cr:
The equivalent mass of an element in a redox reaction is given by its atomic mass divided by the number of electrons gained or lost per atom (which is the change in oxidation state per atom).
Let 'M' be the atomic mass of Cr.
Equivalent mass of Cr = Change in oxidation state per Cr atomAtomic Mass of Cr
Equivalent mass of Cr = 3M
The equivalent mass of Cr is M/3, where M is the atomic mass of Chromium.
