Question
Question: Matrix Match Type Quest 6. Column (I) ...
Matrix Match Type Quest
Column (I)
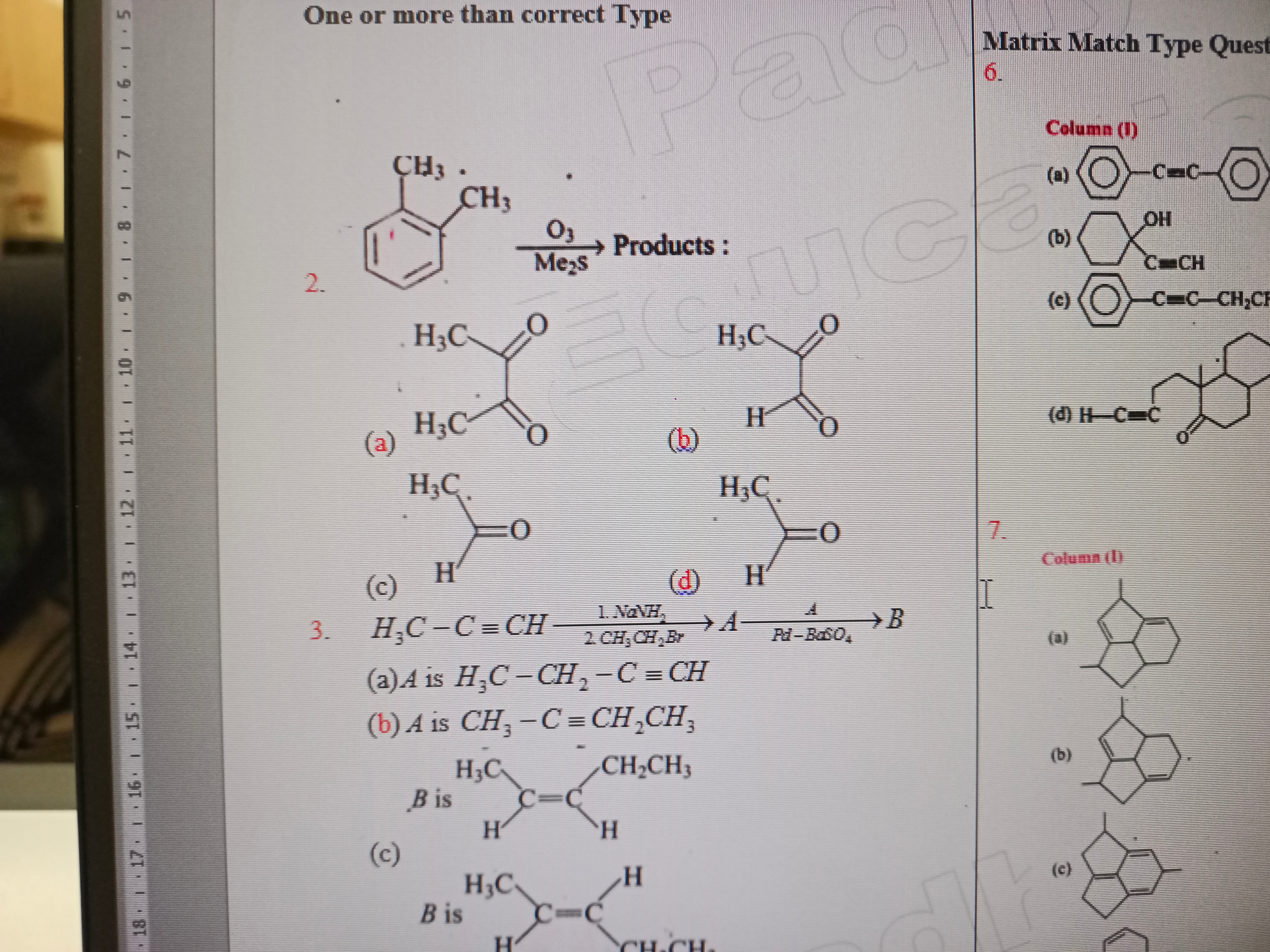
For Question 2: (a) For Question 3: (c)
Solution
The given image contains two organic chemistry problems (2 and 3) to be solved. Both are of "One or more than correct Type".
Problem 2: Ozonolysis of o-xylene
Reactant: o-xylene (1,2-dimethylbenzene)
SMILES: Cc1ccccc1C
Reaction: Ozonolysis (O₃ followed by Me₂S workup)
Mechanism: Ozonolysis of aromatic compounds breaks the carbon-carbon double bonds in the ring and converts the carbon atoms involved in these bonds into carbonyl groups (aldehydes or ketones). For substituted benzenes, the products depend on the positions of the substituents. We need to consider all possible Kekulé resonance structures and their cleavage patterns.
Kekulé Structure 1: If the double bonds are considered between C1-C2, C3-C4, and C5-C6 (where C1 and C2 are substituted with methyl groups):
- Cleavage of the C1=C2 bond (between two methyl-substituted carbons):
CH3-C(=O)-C(=O)-CH3(2,3-butanedione or diacetyl) - Cleavage of the C3=C4 bond (between two unsubstituted carbons):
H-C(=O)-C(=O)-H(glyoxal) - Cleavage of the C5=C6 bond (between two unsubstituted carbons):
H-C(=O)-C(=O)-H(glyoxal)
Kekulé Structure 2: If the double bonds are considered between C1-C6, C2-C3, and C4-C5 (where C1 and C2 are methyl-substituted, and C3, C4, C5, C6 are unsubstituted):
- Cleavage of the C1=C6 bond (between a methyl-substituted and an unsubstituted carbon):
CH3-C(=O)-C(=O)-H(methylglyoxal or pyruvaldehyde) - Cleavage of the C2=C3 bond (between a methyl-substituted and an unsubstituted carbon):
CH3-C(=O)-C(=O)-H(methylglyoxal or pyruvaldehyde) - Cleavage of the C4=C5 bond (between two unsubstituted carbons):
H-C(=O)-C(=O)-H(glyoxal)
Possible Products: Based on both Kekulé structures, the products of ozonolysis of o-xylene are:
CH3-C(=O)-C(=O)-CH3(2,3-butanedione)H-C(=O)-C(=O)-H(glyoxal)CH3-C(=O)-C(=O)-H(methylglyoxal)
Checking the given options:
(a) H3C-C(=O)-C(=O)-CH3 (2,3-butanedione): This is a possible product.
(b) H3C-C(=O)-H (Acetaldehyde): This is a monocarbonyl compound and not a product of ring cleavage.
(c) H-C(=O)-H (Formaldehyde): This is a monocarbonyl compound and not a product of ring cleavage.
(d) H3C-C(=O)-H (Acetaldehyde): Same as (b), not a product.
Therefore, among the given options, only (a) is a correct product of the ozonolysis of o-xylene.
Problem 3: Reaction sequence of Propyne
Starting Material: H3C-C≡CH (Propyne)
Step 1: Formation of A
H3C-C≡CH --(1. NaNH2 / 2. CH3CH2Br)--> A
NaNH2(Sodium amide): This is a strong base. It deprotonates the terminal alkyne to form an acetylide anion.H3C-C≡CH + NaNH2 → H3C-C≡C⁻ Na⁺ + NH3(Propynide anion)CH3CH2Br(Ethyl bromide): The propynide anion acts as a nucleophile and undergoes an SN2 reaction with ethyl bromide, extending the carbon chain.H3C-C≡C⁻ + CH3CH2-Br → H3C-C≡C-CH2CH3 + Br⁻So, A isH3C-C≡C-CH2CH3(Pent-2-yne). SMILES:CCC#CC
Step 2: Formation of B
A --(Pd-BaSO4)--> B
A is Pent-2-yne (H3C-C≡C-CH2CH3).
Pd-BaSO4 (Lindlar's catalyst): This is a poisoned palladium catalyst used for the partial hydrogenation of alkynes to cis-alkenes. It adds hydrogen syn-selectively across the triple bond.
H3C-C≡C-CH2CH3 --(H2, Pd-BaSO4)--> H3C-CH=CH-CH2CH3
The product B is cis-pent-2-ene.
Checking the given options:
(a) A is H3C-CH2-C≡CH (Pent-1-yne): This is incorrect. A is Pent-2-yne.
(b) A is CH3-C≡CH3 (But-2-yne): This is incorrect. A is Pent-2-yne.
(c) B is (structure shown for cis-pent-2-ene):
CH3 CH2CH3 \ / C = C / \ H H
This matches cis-pent-2-ene. This option is correct.
(d) B is (structure shown for trans-pent-2-ene):
CH3 H \ / C = C / \ H CH2CH3
This is trans-pent-2-ene, which would be formed by reduction with Na/liquid NH3 (Birch reduction), not Lindlar's catalyst. This option is incorrect.
Therefore, among the given options, only (c) is correct for problem 3.
Final Answer:
For Question 2: The correct option is (a).
For Question 3: The correct option is (c).
The final answer is 2:(a);3:(c)
Explanation of the solution:
-
Question 2: Ozonolysis of o-xylene (1,2-dimethylbenzene) involves the cleavage of all double bonds in the aromatic ring. Considering the two Kekulé resonance structures, the possible dicarbonyl products are 2,3-butanedione (
CH3-C(=O)-C(=O)-CH3), methylglyoxal (CH3-C(=O)-C(=O)-H), and glyoxal (H-C(=O)-C(=O)-H). Among the given options, only 2,3-butanedione (option a) is a correct product. Acetaldehyde and formaldehyde are not formed from the ring cleavage. -
Question 3: The reaction sequence starts with propyne (
H3C-C≡CH).- Treatment with
NaNH2deprotonates the terminal alkyne to form the propynide anion (H3C-C≡C⁻). - Reaction with
CH3CH2Br(ethyl bromide) is an SN2 reaction, where the propynide anion acts as a nucleophile, leading to the formation ofA, which isH3C-C≡C-CH2CH3(pent-2-yne). - Hydrogenation of
A(pent-2-yne) withPd-BaSO4(Lindlar's catalyst) is a partial and stereoselective hydrogenation that yields a cis-alkene. Therefore,Biscis-pent-2-ene(H3C-CH=CH-CH2CH3). Comparing with the options, (c) correctly depictscis-pent-2-eneas productB. Options (a) and (b) are incorrect forA, and (d) depictstrans-pent-2-ene, which is not formed by Lindlar's catalyst.
- Treatment with
