Question
Question: One of the resonating structures of \(SO_4^{ - 2}\) is as shown. Which set of formal charge on oxyge...
One of the resonating structures of SO4−2 is as shown. Which set of formal charge on oxygen and bond order is correct?
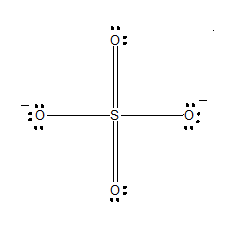
(A) -0.5 and 1.5
(B) 1.5 and 3
(C) 2 and 3
(D) 1.5 and 1.5
Solution
To solve this question we should know the formula to calculate set formal charge and bond order as well as the theory behind it. The formal charge on oxygen in SO4−2 is equal to the formal charge on each oxygen and takes an average of all.
Complete step by step answer:
The formal charge is the number of charges on an atom in a molecule, under assumption that electrons in all bonds are equally shared irrespective of the relative electronegativity.
Formula of formal charges:
Formal charge = number of valence electron in free atom − number of lone pair electrons − 21number of bond pair electrons.
Carefully refer to the diagram, where each oxygen is numbered.
-The formal charges of oxygen 1:
Formal charge = number of valence electron in free atom − number of lone pair electrons − 21number of bond pair electrons.
= 6 - 4 - 2
= 0
-The formal charges of oxygen 2:
Formal charge = number of valence electron in free atom − number of lone pair electrons − 21number of bond pair electrons.
= 6 - 6 - 1
= -1
-The formal charges of oxygen 3:
Formal charge = number of valence electron in free atom − number of lone pair electrons − 21number of bond pair electrons.
= 6 - 4 - 2
= 0
-The formal charges of oxygen 4:
Formal charge = number of valence electron in free atom − number of lone pair electrons − 21number of bond pair electrons.
= 6 - 6 - 1
= -1
The total formal charges on oxygen in SO4−2 is:
Formal charges in oxygen =Total number of oxygensum of formal charge on oxygen 1,2,3 and 4
=40+(−1)+0+(−1)
= - 0.5
Thus, formal charge on SO4−2 is - 0.5
Bond order is the number of chemical bonds present between the pairs of atoms
bond order=Total number of atom sharing the bondnumber of bonds
=46
= 1.5
So, the correct answer is “Option A”.
Note: Generally, Formula to calculate bond pair:
Bond order =21(Nb−Na)
Where, Nb = number of bonding electrons
Na = number of antibonding electrons
But for compound that has resonating structures, then the formula will be
bond order=Total number of atom sharing the bondnumber of bonds
