Question
Question: One of the resonating structure of \[{\text{S}}{{\text{O}}_{\text{4}}}^{{\text{ - 2}}}\] is as shown...
One of the resonating structure of SO4 - 2 is as shown. Which set formal charge on oxygen and bond order is correct?
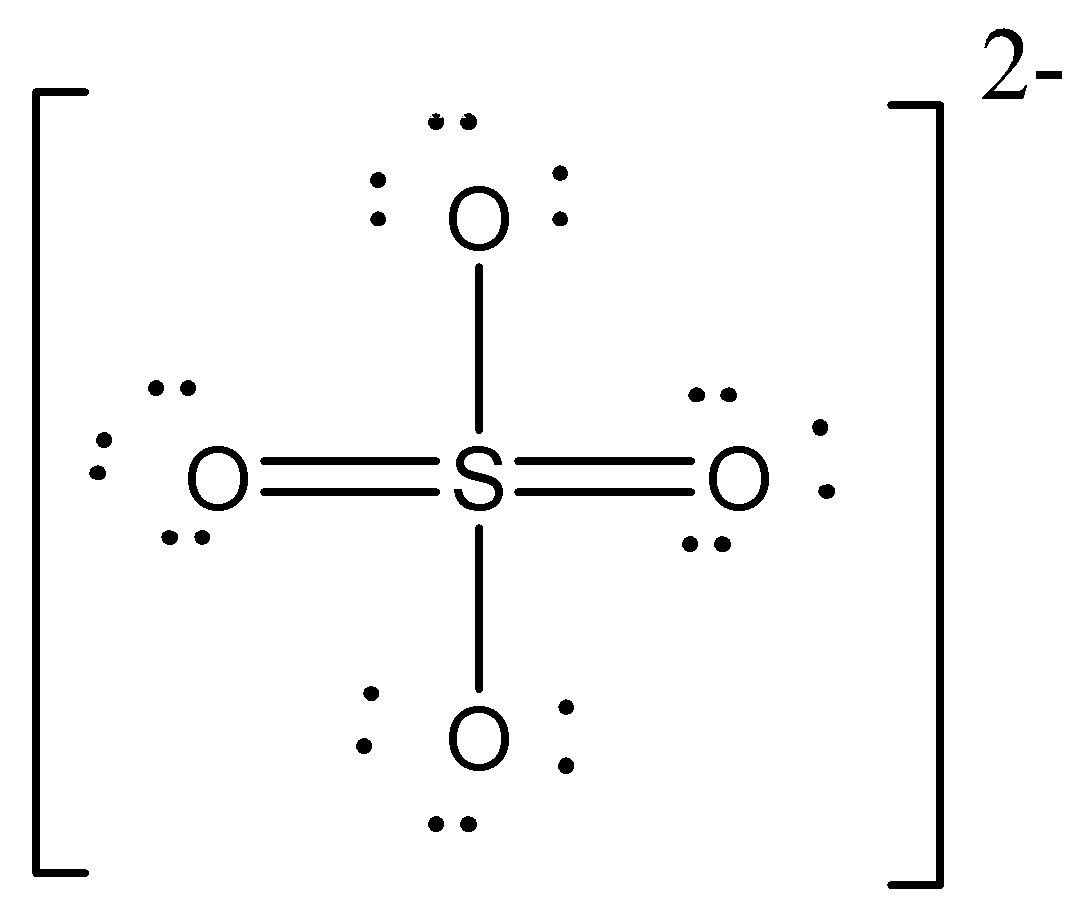
A. −0.5 and 1.5
B. 1.5 and 3
C. 2 and 3
D. 1.5 and 1.5
Solution
Resonating Structures are different structures which shows the possibility of how the bonds may exist in several combinations. SO4 - 2, which is having a total of 6 resonating structure.
Complete step by step answer:
Let’s start with discussing the resonating structures, Resonating Structures are different structures which shows the possibility of how the bonds may exist in several combinations. Since we can’t see the bonds themselves, we can always make some assumptions on how the bonds might exist. More the resonating structures are stronger the stronger the two molecules are bonded. Resonance structures are also called resonance hybrids in valence bond theory.
We are given with SO4 - 2, which is having a total of 6 resonating structure. The resonating structures are given below:
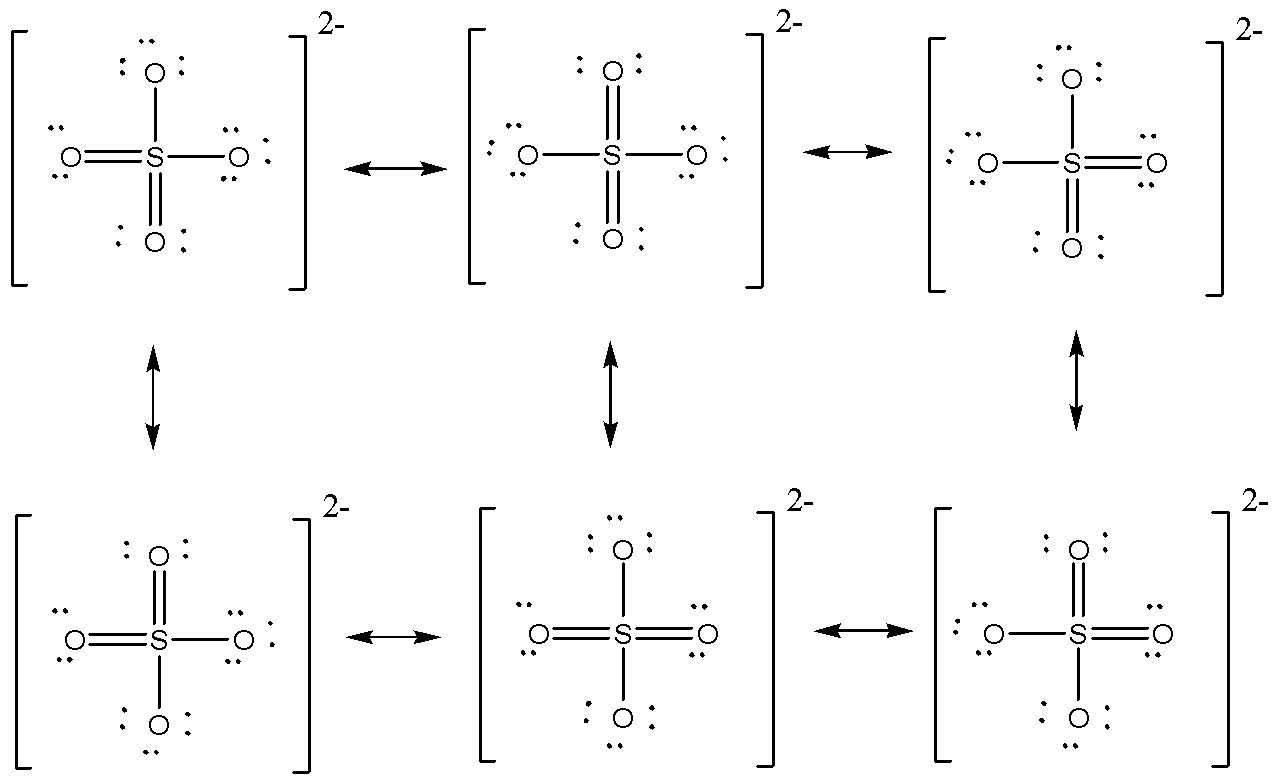
So let’s check the formal charge on first (1), which will be equal to 6Sum of charges on all six structures=6−1+0−1+0−1+0=−0.5,
Hence formal charge on 1st is −0.5.
Let’s check for bond order, which will be equal to
61+2+1+2+1+2=23=1.5
Hence the answer to this question is option A. −0.5 and 1.5.
So, the correct answer is Option A .
Note:
SO4 - 2 ion popularly known as sulphate is present in compounds like sulphuric acid and in many other such compounds. Sulphuric acid with chemical formula H2SO4 is one of the most acidic acid and is used in various industries. This chemical is used in manufacturing of fertilizers, pigments, drugs, explosives, etc.
