Question
Question: One mole of \({{\rm{N}}_{\rm{2}}}{{\rm{H}}_{\rm{4}}}\) loses \(10\) moles of electrons to form a new...
One mole of N2H4 loses 10 moles of electrons to form a new compound A. Assuming that all the nitrogen appears in the new compound, what is the oxidation state of nitrogen in A?
[There is no change in the oxidation state of hydrogen]
A. +1
B. −3
C. +3
D. +5
Solution
As we know that, hydrazine is a neutral compound each hydrogen is in the +1 oxidation state which would put each nitrogen in the −2 state. The sum of the oxidation numbers of N and H has to be zero in a compound.
Step by step answer: When we draw the structure of N2H4 with each nitrogen having a lone pair of electrons and three unpaired electrons, then we showed one nitrogen connected to the other with a single a bonds to each of two hydrogen atoms. Each nitrogen has an octet, each hydrogen has a doublet, the nitrogen shares their bonding electrons equally, but the more electronegative atom, nitrogen controls each of the electrons of the two hydrogen atoms to which each nitrogen is bonded. Hence, this diagram supports the assignment of +1 as the oxidation number of N2H4.
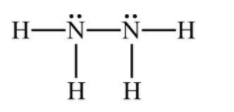
According to the question, one mole of N2H4 loses 10 moles of electron →A.
We know that the oxidation state of H remains the same , according to the question.
∴electron must be lost from nitrogen
1moleN2H4contain2molesofN→2molesofe−
∴ 1 moles of loses 5 moles of e−.
The oxidation state of nitrogen in N2H4 is, first we let the oxidation state of nitrogen is x.
N2H4=0 2x+4(1)=0 ⇒2x+4=0 ⇒2x=−4
On further simplifying the above calculation,
x=−2
thenfinaloxidationstateofNinN2H4=−2
When it losses 5 moles of e−.
thenfinaloxidationstateeachN=−2+5 ⇒thenfinaloxidationstateeachN=+3
Therefore, the correct answer is C.
Note: we know about the oxidation number; it is also called oxidation state. It is the total number of electrons that one atom either gains or loses in order to form a chemical bond with another atom.
