Question
Question: One mole of ethylene glycol was dissolved in 1 kg water and the resulting solution was cooled to -6°...
One mole of ethylene glycol was dissolved in 1 kg water and the resulting solution was cooled to -6°C. How many grams of ice is separated in the process? [Kf of H2O=1.86Kkgmol−1]
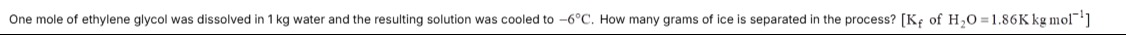
Answer
690
Explanation
Solution
The solution freezes at -6°C. The freezing point depression is 6°C. Using ΔTf=Kf⋅m, the molality of the solution at -6°C is m=1.866. Since the moles of solute (1 mole) remain constant, the mass of water remaining (Wremaining in kg) can be found using m=Wremaining1. Solving for Wremaining, we get Wremaining=61.86=0.31 kg = 310 g. The initial mass of water was 1000 g. The mass of ice separated is 1000 g−310 g=690 g.
