Question
Chemistry Question on Thermodynamics
One mole of an ideal monoatomic gas undergoes two reversible processes (A→B and B→C) as shown in the given figure:

A→B is an adiabatic process. If the total heat absorbed in the entire process (A→B and B→C) is RT2 ln 10, the value of 2log V3 is ___ . [Use, molar heat capacity of the gas at constant pressure, Cp,m=25R]
Answer

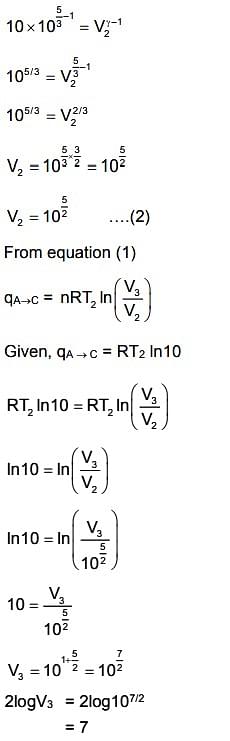
the correct answer is 7
