Question
Question: One mole of an ideal monoatomic gas is taken through a thermodynamic process shown in the P – V diag...
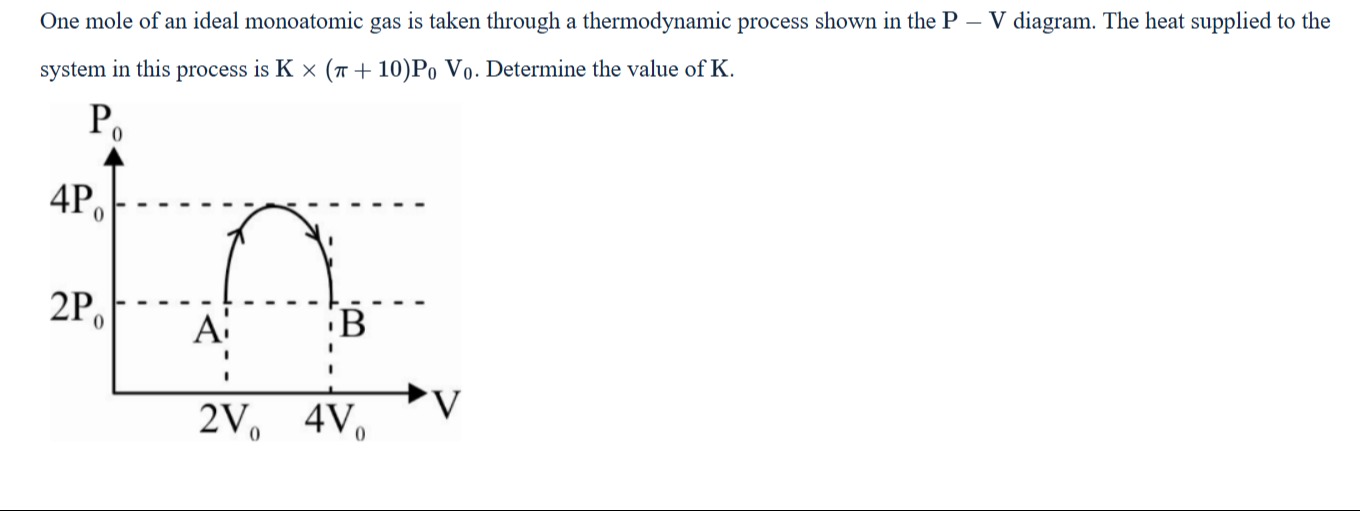
One mole of an ideal monoatomic gas is taken through a thermodynamic process shown in the P – V diagram. The heat supplied to the system in this process is K × (π + 10)P0V0. Determine the value of K.
1
Solution
The process is a cycle A → B → A.
Process A to B is isobaric expansion at P=2P0 from V=2V0 to V=4V0. Work done WAB=2P0(4V0−2V0)=4P0V0. Change in internal energy ΔUAB=nCvΔTAB. TA=R(2P0)(2V0)=R4P0V0, TB=R(2P0)(4V0)=R8P0V0. ΔTAB=R4P0V0. For monoatomic gas, Cv=23R. ΔUAB=1×23R×R4P0V0=6P0V0. Heat supplied QAB=ΔUAB+WAB=6P0V0+4P0V0=10P0V0.
Process B to A is a curved path from (4V0,2P0) to (2V0,2P0) passing through (3V0,4P0). This is the upper half of an ellipse centered at (3V0,2P0) with semi-axes a=V0 and b=2P0. Work done WBA=∫4V02V0PdV. The area under the curve is the area of the rectangle with height 2P0 and width 2V0 plus the area of the semi-ellipse with semi-axes a=V0 and b=2P0. The area of the semi-ellipse is 21πab=21π(V0)(2P0)=πP0V0. The area under the curve from V=4V0 to V=2V0 is the area of the rectangle from V=2V0 to V=4V0 at P=2P0 plus the area of the semi-ellipse above P=2P0. The area under the curve is 2P0(4V0−2V0)+πP0V0=4P0V0+πP0V0. Since the volume is decreasing, the work done by the system is negative. WBA=−(4P0V0+πP0V0)=−4P0V0−πP0V0. Change in internal energy ΔUBA=nCvΔTBA. ΔTBA=TA−TB=−R4P0V0. ΔUBA=1×23R×(−R4P0V0)=−6P0V0. Heat supplied QBA=ΔUBA+WBA=−6P0V0+(−4P0V0−πP0V0)=−10P0V0−πP0V0.
The heat supplied to the system in this process (the cycle) is the net heat transfer Qcycle=QAB+QBA=10P0V0+(−10P0V0−πP0V0)=−πP0V0. The given heat supplied is K×(π+10)P0V0. If this is the net heat supplied, then −πP0V0=K(π+10)P0V0, so K=−π+10π.
If the question asks for the magnitude of the heat transferred during the process from B to A, then ∣QBA∣=∣−10P0V0−πP0V0∣=(10+π)P0V0. If ∣QBA∣=K(π+10)P0V0, then (10+π)P0V0=K(π+10)P0V0, so K=1.
Given the structure of the expression, it is highly probable that the question is asking for the magnitude of the heat transferred during the process from B to A.
The final answer is 1.
