Question
Question: One mole of an ideal monoatomic gas is taken along the path ABCA as shown in the PV diagram. The max...
One mole of an ideal monoatomic gas is taken along the path ABCA as shown in the PV diagram. The maximum temperature attained by the gas along the path BC is given by
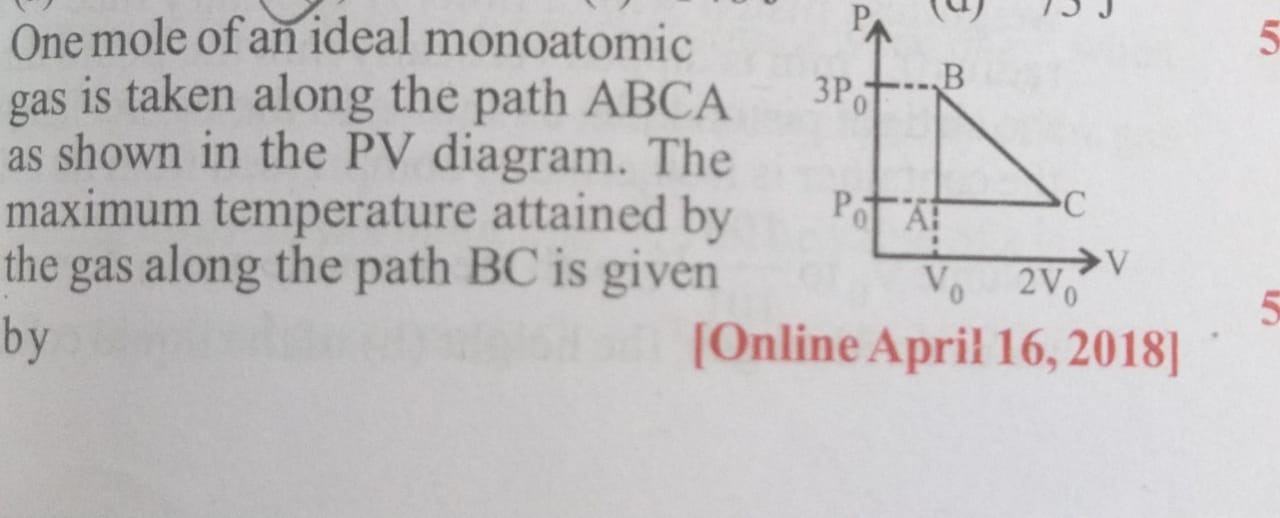
A
8R25P0V0
B
R3P0V0
C
RP0V0
D
2R5P0V0
Answer
8R25P0V0
Explanation
Solution
The temperature T is proportional to the product PV (PV=nRT). For n=1, T=RPV. The path BC is a line segment connecting B(V0,3P0) and C(2V0,P0). The equation of this line is P=5P0−V02P0V. The product PV=V(5P0−V02P0V)=5P0V−V02P0V2. To find the maximum, we set the derivative with respect to V to zero: dVd(PV)=5P0−V04P0V=0, which gives V=45V0. The corresponding pressure is P=5P0−V02P0(45V0)=25P0. The maximum product PVmax=(25P0)(45V0)=825P0V0. Thus, Tmax=RPVmax=8R25P0V0.
