Question
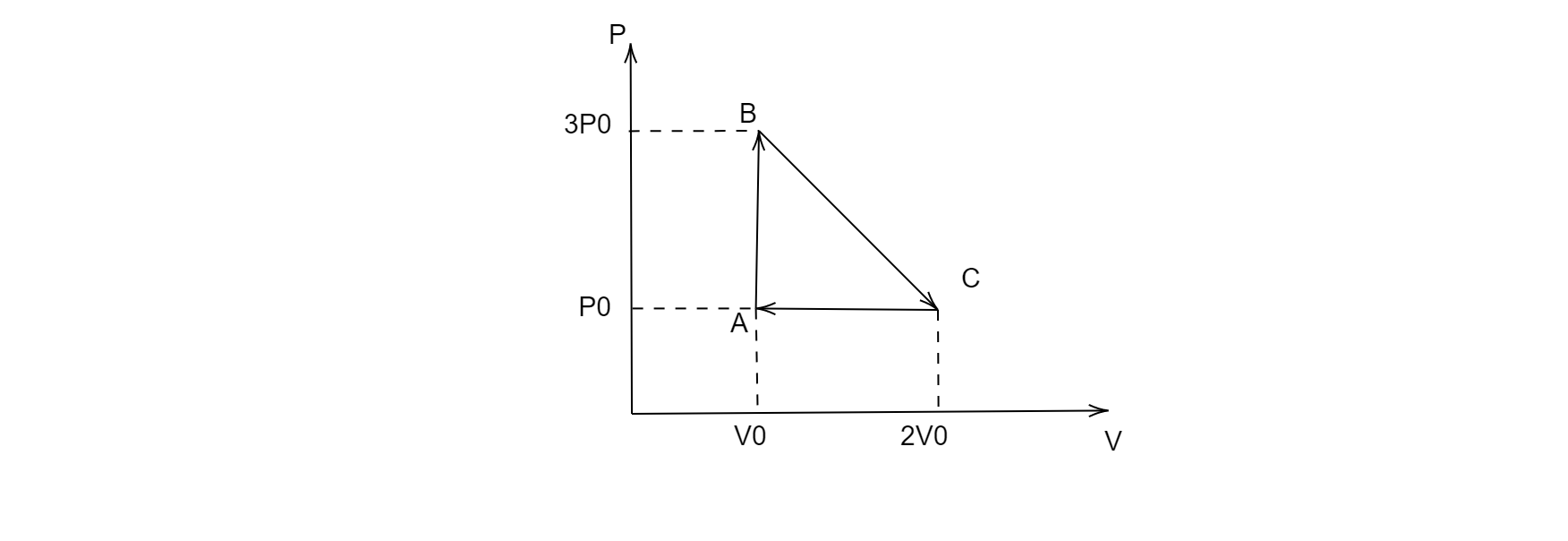
Question: One mole of an ideal monatomic gas is taken round the cyclic process \(ABCA\) as shown in the figure...
One mole of an ideal monatomic gas is taken round the cyclic process ABCA as shown in the figure, calculate the heat rejected by the gas in the path CA and the heat absorbed by the gas in the path AB.
Solution
The use of Cp and Cv will simplify the problem. Also, since CA and AB processes are isobaric and isochoric respectively, equations can be used using these two properties of the gas in the respective paths.
Complete step by step solution:
Since the gas is monatomic, therefore the value of number of moles i.e. n will be 1. The specific heat capacities at constant volume and constant pressure are:
Cv=23R
⇒RCv=23
Cp=25R
⇒RCp=25
Where Cp and Cv are the molar specific heat capacities of the gas at constant pressure and constant volume respectively of an ideal gas. Specific heat capacity of a substance is defined as the heat supplied per unit mass of that particular substance per unit rise in temperature.
Since the process CA is isobaric i.e. the pressure remains constant throughout the process for this path, therefore the heat rejected by the gas in the path CA will be:
⇒dhCA=Cp(Tfinal−Tinitial)
⇒dhCA=Cp(RP0V0−R2P0V0)
⇒dhCA=RCp(P0V0−2P0V0)
⇒dhCA=25(P0V0−2P0V0)
Where dhCA is the heat rejected during the process.
⇒dhCA=−25(P0V0)
Since the process AB is isochoric, i.e. the volume remains constant throughout the process for this path, therefore the heat rejected by the gas in the path AB will be:
⇒dhAB=CvdT
Where dhAB is the heat absorbed by the monatomic gas in the process AB.
⇒dhAB=Cv(Tfinal−Tinitial)
⇒Cv(RPfVf−RPiVi)
⇒RCv(PfVf−PiVi)
⇒23(PfVf−PiVi)
⇒23(3P0V0−P0V0)
⇒3P0V0
Therefore, the heat rejected by the gas in the path CA is −25(P0V0) and the heat absorbed by the gas in the path AB is 3P0V0.
Note: If the heat rejected in the path CA is negative, then this implies that no heat is rejected in this process but there is heat absorption in the process. In the process AB no such negative sign is encountered therefore the process has absorbed heat.
