Question
Question: One mole of an ideal monatomic gas is taken round the cyclic process ABCA as shown in figure. Calcul...
One mole of an ideal monatomic gas is taken round the cyclic process ABCA as shown in figure. Calculate –
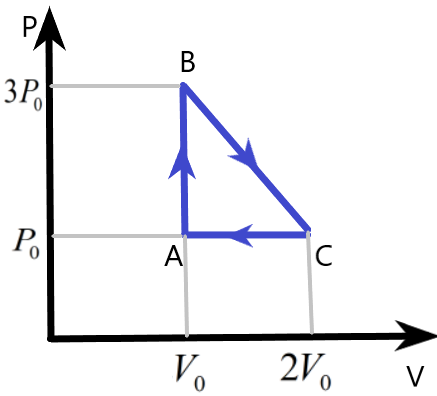
(a) The work done by the gas
(b) The heat rejected by the gas in the path CA and the heat absorbed by the gas in the path AB.
(c) The net heat absorbed by the gas in the path BC.
Solution
We need to understand the relation between the pressure and volume variation in a cyclic process of a monatomic gases with the heat absorption and the heat rejection of the system and the net work done in order to solve this problem.
Complete answer:
We are given a cyclic process of an ideal monatomic gas with the help of a diagram. The pressure and volume variation of the system is plotted which can be used to find the other parameters involved in this system during the process.
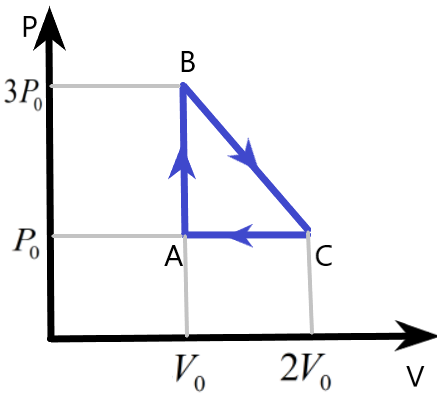
We know that the work done by the gas in the cyclic process can be easily found by finding the area under the pressure – volume curve of the process. Here, the area of the triangle ABC will give us the work done W, as –
