Question
Question: One mole of an ideal monatomic gas is taken along the path ABCA as shown in the PV diagram. The maxi...
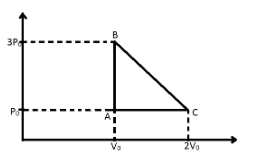
One mole of an ideal monatomic gas is taken along the path ABCA as shown in the PV diagram. The maximum temperature attained by the gas along the path BC is given by:-
A. 825RP0V0
B. 425RP0V0
C. 1625RP0V0
D. 85RP0V0
Solution
An ideal gas equation is given as PV=nRT. We can write the PV equation for the process BC using the equation of straight line y−y1=x2−x1y2−y1(x−x1), where (x1,y1)and(x2,y2) are known points B and C. We can convert this PV equation in terms of temperature T using the ideal gas equation. For maximum temperature dVdT=0.
Complete step by step answer:
We know that an ideal gas equation is given as PV=nRT. Therefore temperature T=nRPV.
Given, number of moles, n=1.
Temperature of point A=R3P0V0
Temperature off point B=RP02V0
Now we write the PV equation for the process BC using the equation of a straight line with two given point.
P - 3{P_0} = \dfrac{{{P_0} - 3{P_0}}}{{2{V_0} - {V_0}}}\left( {V - {V_0}} \right) \\\
\Rightarrow P - 3{P_0} = \dfrac{{ - 2{P_0}}}{{{V_0}}}\left( {V - {V_0}} \right) \\\
\Rightarrow P = \dfrac{{ - 2{P_0}V}}{{{V_0}}} + 5{P_0} \\\
Multiplying the equation with V.
PV=V0−2P0V2+5P0V
Replacing PV with RT.
RT=−V02P0V2+5P0V
For maximum temperature dVdT=0.
Therefore we get the maximum temperature t as
∴T=825RP0V0
Note: It is important to know the equation for ideal gas to solve this question (PV=nRT). We find the maximum or minimum value of any quantity using differentiation.For the net work involved in a cyclic process is the area enclosed in a P-V diagram, if the cycle goes clockwise, the system does work and if the cycle goes anticlockwise, then work is done on the system.
