Question
Question: One mole of an ideal gas with the adiabatic exponent \(\gamma \) goes through the polytropic process...
One mole of an ideal gas with the adiabatic exponent γ goes through the polytropic process as a result of which the absolute temperature of the gas increases τ fold. The polytropic constant equals n. Find the entropy of the gas in this process.
(A) (n−1)(γ−1)(n−γ)Rlnτ
(B) (n−γ)(n−γ)Rlnτ
(C) (n−1γ)(γ−1)(n−γ)Rlnτ
(D) (n−1)γ(n−γ)Rlnτ
Solution
Entropy is a term in Physics that is associated with the randomness or disorderness of a particular process. In simple words, it can be explained as the heat or thermal energy that is not useful for any work, per unit temperature.
Formula used:
The formula to calculate entropy is given as
∫dS=∫TdQ
Also, the relation between dQ and T is given by,
dQ=nCdT
For a polytropic process, the formula for molar heat capacity is given as,
C=(γ−1R−n−1R)
Complete step by step solution:
Let the initial temperature be Ti.
It is given that the absolute temperature increases by τ fold.
If the final temperature is Tf , then we can say that,
Tf=τTi
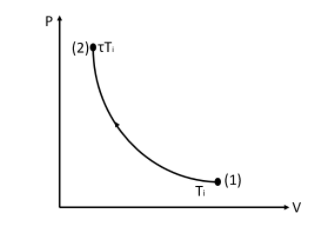
This is the P−V curve for an increase in temperature for an adiabatic process.
From the figure, we can see that the temperature from point (1) has increased to τ times the initial temperature at point (2).
At point (2) the pressure increases and the volume decreases.
We know that the randomness increases with the decrease in volume.
Therefore we can say that the entropy increases at point (2).
The formula to calculate entropy is given as
∫dS=∫TdQ
where, dS is the change in entropy,
dQ is the heat energy released, and
T is the temperature.
∴ΔS=∫TdQ ……….(I)
Also, the relation between dQ and T is given by,
dQ=nCdT
where, n is the number of moles, and
C is the molar heat capacity.
The value of C can be found using specific heat capacity, or heat capacity per unit mass, c=mC.
From the question, we have, n as one mole.
∴dQ=CdT
Substituting this term in the equation (I) we get,
ΔS=∫TCdT
According to the requirement of the question, if we integrate the above equation from Ti to Tf we get, ΔS=Ti∫TfTCdT
Upon solving the integration we get,
ΔS=ClnT]TiTf
Substituting the values of the integrating limits we get,
ΔS=C(lnτTi−lnTi)
Using the property of logarithm, lnA−lnB=lnBA we get,
ΔS=ClnTiτTi
Cancelling Ti from numerator and denominator we get,
ΔS=Clnτ ……….(II)
For a polytropic process, the formula for molar heat capacity is given as,
C=(γ−1R−n−1R)
where, n is given in the question as the polytropic constant,
R is the Rydberg’s constant, and
γ is the adiabatic exponent.
Substituting this value in the equation (II) we get,
ΔS=(γ−1R−n−1R)lnτ
Taking R common,
ΔS=R(γ−11−n−11)lnτ
⇒ΔS=R[(n−1)(γ−1)(n−1)−(γ−1)]lnτ
Upon solving the numerator we get,
ΔS=R[(n−1)(γ−1)n−γ]lnτ
⇒ΔS=(n−1)(γ−1)(n−γ)Rlnτ
Hence the correct answer is option (A) (n−1)(γ−1)(n−γ)Rlnτ.
Note: The adiabatic exponent, or the heat capacity ratio, or Laplace’s coefficient γ is equal to the ratio between the heat capacity at constant pressure, CP and the heat capacity at constant volume, CV. This concept is specially applied for processes involving ideal gases.
