Question
Question: One mole of an ideal gas is taken reversibly from state A to C through path ABC as shown below. If t...
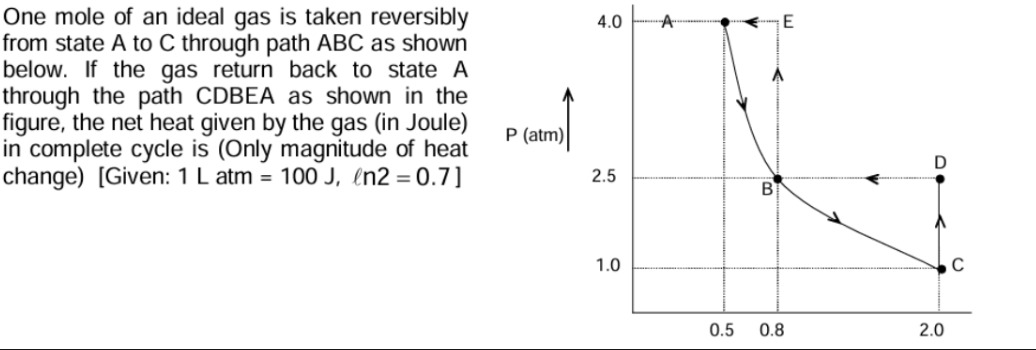
One mole of an ideal gas is taken reversibly from state A to C through path ABC as shown below. If the gas return back to state A through the path CDBEA as shown in the figure, the net heat given by the gas (in Joule) in complete cycle is (Only magnitude of heat change) [Given: 1 L atm = 100 J, ln2=0.7]
140
Solution
The cycle is A → C through path ABC, and then C → A through path CDBEA. The net heat given by the gas in a complete cycle is equal to the net work done by the gas in the cycle, i.e., Qcycle=Wcycle. The net work done in the cycle is the sum of the work done in each process. Wcycle=WABC+WCDBEA.
First, let's find the work done in path ABC. From the figure, the points A, B, and C have coordinates: A: (V=0.5 L, P=4.0 atm) B: (V=0.8 L, P=2.5 atm) C: (V=2.0 L, P=1.0 atm) Let's check the product PV for these points: PAVA=4.0 atm×0.5 L=2.0 L atm PBVB=2.5 atm×0.8 L=2.0 L atm PCVC=1.0 atm×2.0 L=2.0 L atm Since PV is constant along the path ABC, the path ABC is an isothermal process. The work done in an isothermal process for an ideal gas is given by W=∫ViVfPdV. Since PV=constant=2.0, P=V2.0. WABC=∫VAVCV2.0dV=2.0[lnV]VAVC=2.0(lnVC−lnVA)=2.0ln(VAVC). WABC=2.0ln(0.52.0)=2.0ln(4)=2.0×2ln(2)=4.0ln(2). Given ln2=0.7, WABC=4.0×0.7=2.8 L atm. Converting to Joules, WABC=2.8 L atm×100L atmJ=280 J.
Next, let's find the work done in path CDBEA. This path consists of four segments: C to D, D to B, B to E, and E to A. The coordinates of the points are: C: (V=2.0 L, P=1.0 atm) D: (V=2.0 L, P=2.5 atm) B: (V=0.8 L, P=2.5 atm) E: (V=0.8 L, P=4.0 atm) A: (V=0.5 L, P=4.0 atm)
Path C to D: Isochoric process (constant volume, V=2.0 L). WCD=∫VCVDPdV=0 since dV=0. Path D to B: Isobaric process (constant pressure, P=2.5 atm). WDB=∫VDVBPdV=P(VB−VD)=2.5 atm×(0.8−2.0) L=2.5×(−1.2) L atm=−3.0 L atm. Path B to E: Isochoric process (constant volume, V=0.8 L). WBE=∫VBVEPdV=0 since dV=0. Path E to A: Isobaric process (constant pressure, P=4.0 atm). WEA=∫VEVAPdV=P(VA−VE)=4.0 atm×(0.5−0.8) L=4.0×(−0.3) L atm=−1.2 L atm.
The total work done in path CDBEA is WCDBEA=WCD+WDB+WBE+WEA=0+(−3.0)+0+(−1.2)=−4.2 L atm. Converting to Joules, WCDBEA=−4.2 L atm×100L atmJ=−420 J.
The net work done in the cycle is Wcycle=WABC+WCDBEA=280 J+(−420) J=−140 J. The net heat given by the gas in the complete cycle is Qcycle=Wcycle=−140 J. The negative sign indicates that the net heat is given out by the gas. The question asks for the magnitude of the net heat given by the gas. Magnitude of heat change = ∣−140 J∣=140 J.
