Question
Question: One mole of an ideal gas is taken from ‘a to b’ along two paths denoted by the solid and the dashed ...
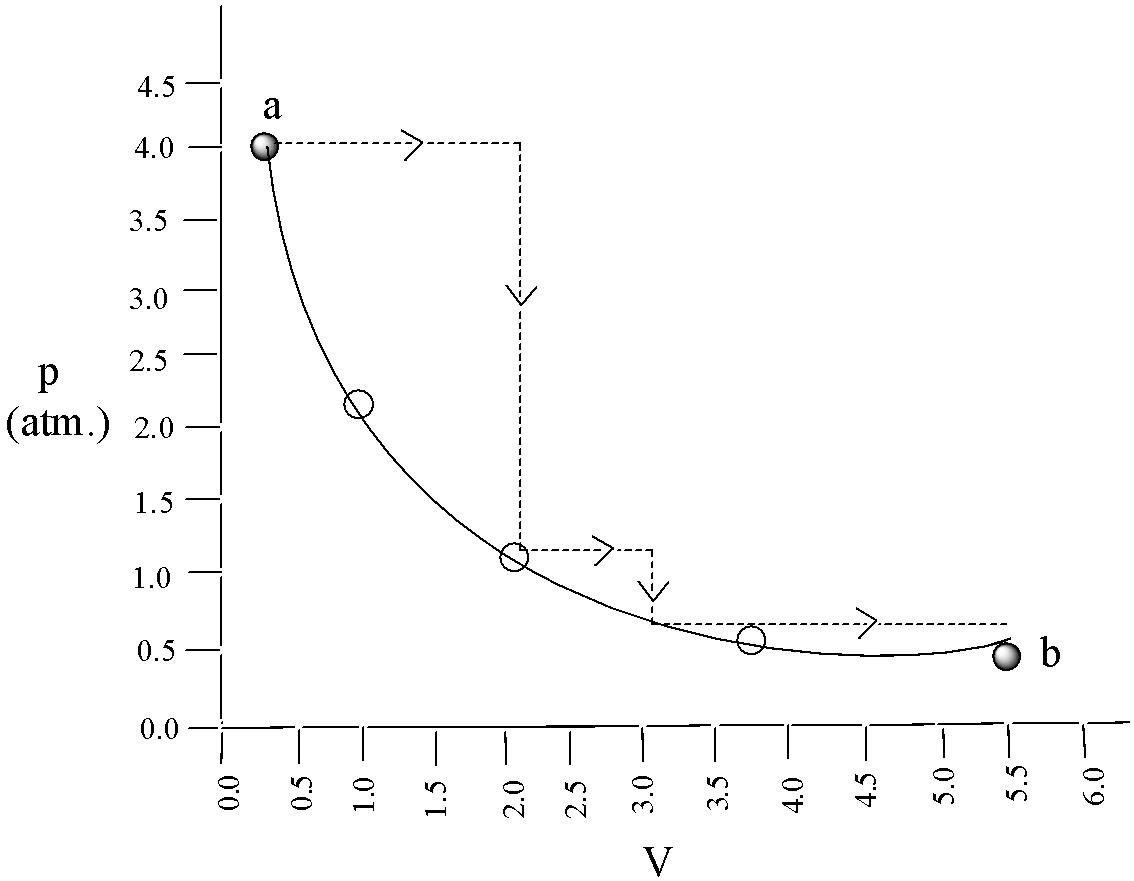
One mole of an ideal gas is taken from ‘a to b’ along two paths denoted by the solid and the dashed lines as shown in the graph below. If the work done along the solid line path is WSand that along the dotted line path is Wd. Then the integer closest to the ratio Wd/Wsis,
Solution
We need to know that in the case of ideal gas, there are so many randomly moving point particles and it is not undergoing any interparticle interactions. And the ideal gases should obey the ideal gas law and it follows some rules. That is, the molecules present in the ideal gas, which does not attract or repels each other. And there is only an elastic collision between the ideal gas molecules.
Complete answer:
So many thermodynamic processes are present in a system and all the processes have unique properties. According to the question, the given system is in the form of isothermal reversible expansion process. And there is a graphical representation of the area under the PV – curve.
Given, Ws represents the work done along the solid path and Wd is the work done along the dotted line in the graph.
By using the graphical values,
Wd=4×1.5+1×1+2.5×32
⇒Wd=8.65
The given process is an isothermal reversible expansion,
Thus, Ws=−2.303nRTlogv1v2
From the plot, will get the value of v2 is equal to 5.5 and v1 equal to 0.2
Hence, Ws=−2.303nRTlog0.25.5
WS=4.79
Therefore, the integer close to the ratio is equal to,
WsWd=4.798.65=1.80≃2
Note:
We have to know that in the case of isothermal reversible expansion, the volume is slowly increasing in the presence of a constant temperature. In the case of an isothermal reversible expansion process, the expansion of PV work done was possible by the absorption of heat reversibly into the ideal gas. And they do not lose the heat. But the change in internal energy will be equal to zero.
