Question
Question: One mole of an ideal diatomic gas undergoes a process in which the gas pressure relates to its tempe...
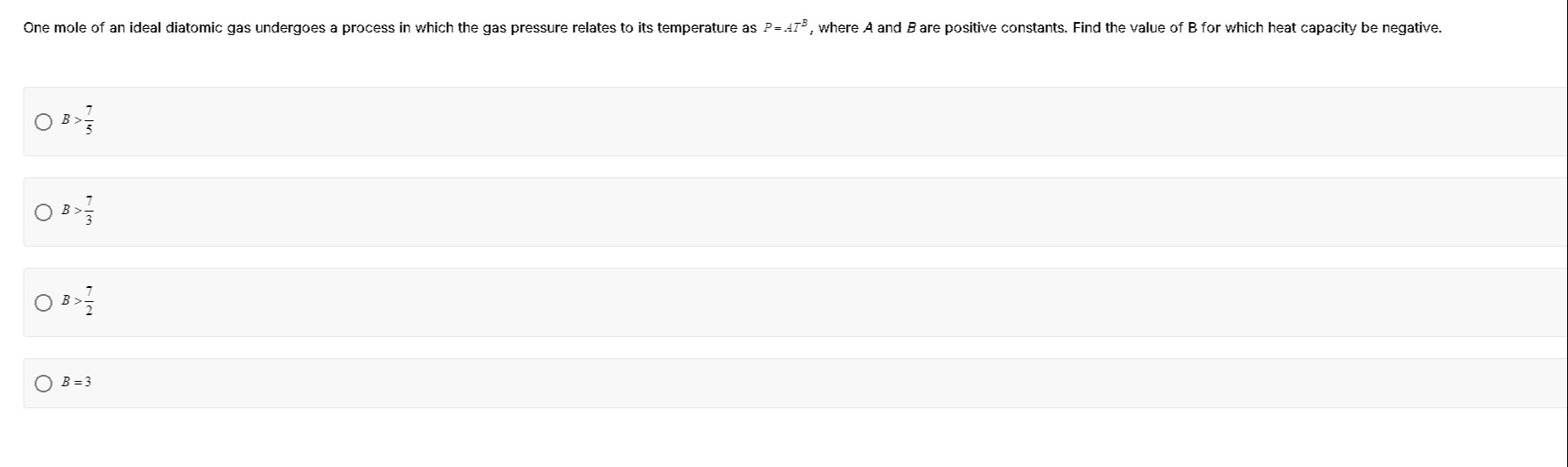
One mole of an ideal diatomic gas undergoes a process in which the gas pressure relates to its temperature as P=ATB, where A and B are positive constants. Find the value of B for which heat capacity be negative.
A
B>57
B
B>37
C
B>27
D
B=3
Answer
B>27
Explanation
Solution
For one mole of an ideal diatomic gas, the internal energy change is
dU=CVdT=25RdT.Using the ideal gas law PV=RT and given P=ATB, we obtain
V=PRT=ART1−B.Differentiate with respect to T:
dV=AR(1−B)T−BdT.Thus, the work done is
dW=PdV=ATB⋅AR(1−B)T−BdT=R(1−B)dT.The heat added is
dQ=dU+dW=(25R+R(1−B))dT=R(27−B)dT.The effective heat capacity for the process is
C=dTdQ=R(27−B).For the heat capacity to be negative,
27−B<0⟹B>27.