Question
Question: One mole of an ideal diatomic gas is taken through the cycle as shown in the figure: \[1 \to 2\]:...
One mole of an ideal diatomic gas is taken through the cycle as shown in the figure:
1→2: isochoric process
2→3: a straight line of P-V diagram
3→1: isobaric process
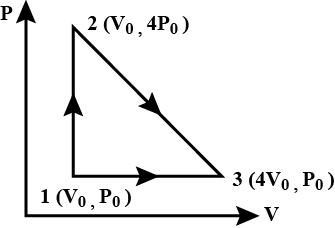
The average molecular speed of the gas in the states 1, 2 and 3 in the ratio:
A. 1:2:2
B. 1:2:2
C. 1:1:1
D. 1:2:4
Solution
In 1→2, Pressure is directly proportional to temperature. In 3→1, Volume is directly proportional to Temperature. And the average molecular speed (Vrms) is directly proportional to MRT, hence v∝T
Complete step by step answer:
In the state 1
Let temperature be To
and we know that, PV=nRT
In the state 2
Since 1→2 is an isochoric process
Hence, in State 1
TP= Constant
v∝T0
when Pressure is equal to 4Po. Thus, the Temperature =4To
State 3
Since 3→1 is an isobaric process,
According to Charlee’s law
V∝T
TV= Constant
When Volume is 4Vo. Thus Temperature =4To
The root-mean-square speed or the average molecular speed (Vrms) is directly proportional to MRT
⇒v∝T
The average velocity of the gas molecule has the formula : Vavg=M8RT
Where,
V= molecular speed of the particle
T = Temperature in Kelvin
M = molar mass of the compound
R = Ideal gas constant
Hence,
State 1, v∝T0
State 2, v∝4To=2To
State3, v∝4To=2To
Hence, V1:V2:V3=To:4To:4YTo
Ratio =1:2:2
Therefore, the correct answer is option (A).
Note: Isobaric process is carried out at a constant pressure. In such a process dP=0. Isochoric process is a process in which the volume of the system remains constant, whereby dV=0. According to the Kinetic Molecular Theory of Gases, the molecular speed of the gas explains that gas particles are in continuous motion and they exhibit ideally elastic collisions.
