Question
Question: One mole of a perfect monoatomic gas is put through a cycle consisting of the following three revers...
One mole of a perfect monoatomic gas is put through a cycle consisting of the following three reversible steps:
(CA): Isothermal compression from 2 atm and 10 litres to 20 atm and 1 litre.
(AB): Isobaric expansion to return the gas to the original volume of 10 litres with T going from T1to T2.
(BC): Cooling at constant volume to bring the gas to the original pressure and temperature.
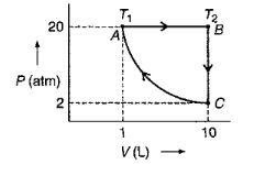
The steps are shown schematically in figure given above
(a) calculate T1to T2.
(b) Calculate ΔU,qandwin calories, for each step and for the cycle.
Solution
For monoatomic gas, degrees of freedom is 3 ∴Cv=23R. We can use Ideal gas equation and the laws of thermodynamics to solve the given question.
Complete step by step answer:
Ideal gas equation, PV=nRT
First law of thermodynamics: ΔU=q+w
We have PV=nRT
Where, P is pressure of ideal gas
V is volume of ideal gas
n is number of molecules
T is temperature
R is the ideal gas constant.
It is given that n=1mole
In step CA:
Let us say, P1V1=nRT1 . . . (1)
P1=2 atm
V1=10litres
∴2×10=1×0.821×T1
∴T1=243.60K
In step BC:
Volume is constant
⇒P2V1=1RT2 …. (2)
Divide equation (1) by equation (2)
P2V1P1V1=RT2RT1
⇒P2P1=T2T1
202=T2243.60
T2=10×243.6
=2436K
Now we have to find ΔUi.e. change in energy
- From A to B
(ΔU)AB=nCvΔT
=1×23R(T2−T1)
=1×23×2×(246−243.6) ∵R=2
=6577.2cal - From B to C
(ΔU)BC=nCvR(T1−T2)
=1×23×2×(243.6−2436)
=−3577.2cal - Process C to A is isothermal process
∴(ΔU)CA=nCv(T1−T1) (As there is no change in temperature.)
=0
Now the ΔUof cycle is
ΔU=(ΔU)AB+(ΔU)BC+(ΔU)CA
=6577.2−6577.2+0
=0
∴Net internal energy of cycle is zero
Now calculating the work done - A to B is isobaric process, so work done is
(W)AB=−PΔV
=−P(VB−VA)
=−20(10−1)
=180L atm
=−180×0.08212cal
=−4384.9cal - Volume is constant from B to C.
∴ΔV=0i.e. change in volume is zero.
⇒WBC=0
Now, for finding qwe use, the 1stlaw of thermodynamics
ΔU=q+w
q=ΔU−w - For A to B
qAB=ΔUAB−WAB
=6577.2−(−4384.9)
=10962.1cal - For B to C
qBC=ΔUBC−WBC
=−6577.2−0
=−6577.2cal - For C to a
qCA=ΔUCA−WCA
=0−1122
=−1122cal
∴For total cycle
qcycle=qAB+qBC+qCA
=10962.1−6577.2−1122
=3262.9cal
Note: Positive sign indicates increase in energy and negative sign indicates the decrease in energy. To solve such types of questions, we need to have a clear idea about in which process temperature is constant and in which process volume is constant etc.
